Uncoupling proteins 2 and 3 are highly active H(+) transporters and highly nucleotide sensitive when activated by coenzyme Q (ubiquinone)
- PMID: 11171965
- PMCID: PMC29271
- DOI: 10.1073/pnas.98.4.1416
Uncoupling proteins 2 and 3 are highly active H(+) transporters and highly nucleotide sensitive when activated by coenzyme Q (ubiquinone)
Abstract
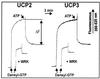
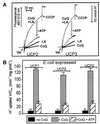
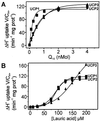
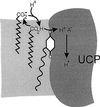
Based on the discovery of coenzyme Q (CoQ) as an obligatory cofactor for H(+) transport by uncoupling protein 1 (UCP1) [Echtay, K. S., Winkler, E. & Klingenberg, M. (2000) Nature (London) 408, 609-613] we show here that UCP2 and UCP3 are also highly active H(+) transporters and require CoQ and fatty acid for H(+) transport, which is inhibited by low concentrations of nucleotides. CoQ is proposed to facilitate injection of H(+) from fatty acid into UCP. Human UCP2 and 3 expressed in Escherichia coli inclusion bodies are solubilized, and by exchange of sarcosyl against digitonin, nucleotide binding as measured with 2'-O-[5-(dimethylamino)naphthalene-1-sulfonyl]-GTP can be restored. After reconstitution into vesicles, Cl(-) but no H(+) are transported. The addition of CoQ initiates H(+) transport in conjunction with fatty acids. This increase is fully sensitive to nucleotides. The rates are as high as with reconstituted UCP1 from mitochondria. Maximum activity is at a molar ratio of 1:300 of CoQ:phospholipid. In UCP2 as in UCP1, ATP is a stronger inhibitor than ADP, but in UCP3 ADP inhibits more strongly than ATP. Thus UCP2 and UCP3 are regulated differently by nucleotides, in line with their different physiological contexts. These results confirm the regulation of UCP2 and UCP3 by the same factors CoQ, fatty acids, and nucleotides as UCP1. They supersede reports that UCP2 and UCP3 may not be H(+) transporters.
Figures

Similar articles
-
Reconstitution of recombinant uncoupling proteins: UCP1, -2, and -3 have similar affinities for ATP and are unaffected by coenzyme Q10.J Biol Chem. 2003 Jul 11;278(28):25825-31. doi: 10.1074/jbc.M302126200. Epub 2003 May 6. J Biol Chem. 2003. PMID: 12734183
-
Activating omega-6 polyunsaturated fatty acids and inhibitory purine nucleotides are high affinity ligands for novel mitochondrial uncoupling proteins UCP2 and UCP3.J Biol Chem. 2003 Jun 6;278(23):20761-9. doi: 10.1074/jbc.M212850200. Epub 2003 Apr 1. J Biol Chem. 2003. PMID: 12670931
-
Coenzyme Q is an obligatory cofactor for uncoupling protein function.Nature. 2000 Nov 30;408(6812):609-13. doi: 10.1038/35046114. Nature. 2000. PMID: 11117751
-
Uncoupling protein, H+ transport and regulation.Biochem Soc Trans. 2001 Nov;29(Pt 6):806-11. doi: 10.1042/0300-5127:0290806. Biochem Soc Trans. 2001. PMID: 11709079 Review.
-
Reconstitution of novel mitochondrial uncoupling proteins UCP2 and UCP3.Biosci Rep. 2002 Feb;22(1):33-46. doi: 10.1023/a:1016009022186. Biosci Rep. 2002. PMID: 12418549 Review.
Cited by
-
UCP2 and ANT differently modulate proton-leak in brain mitochondria of long-term hyperglycemic and recurrent hypoglycemic rats.J Bioenerg Biomembr. 2013 Aug;45(4):397-407. doi: 10.1007/s10863-013-9503-2. Epub 2013 Mar 17. J Bioenerg Biomembr. 2013. PMID: 23504111
-
The Paradox of Coenzyme Q10 in Aging.Nutrients. 2019 Sep 14;11(9):2221. doi: 10.3390/nu11092221. Nutrients. 2019. PMID: 31540029 Free PMC article. Review.
-
Ischemia–reperfusion injury in patients with fatty liver and the clinical impact of steatotic liver on hepatic surgery.Surg Today. 2014 Sep;44(9):1611-25. doi: 10.1007/s00595-013-0736-9. Surg Today. 2014. PMID: 24078000 Review.
-
Uncoupling protein-2 is critical for nigral dopamine cell survival in a mouse model of Parkinson's disease.J Neurosci. 2005 Jan 5;25(1):184-91. doi: 10.1523/JNEUROSCI.4269-04.2005. J Neurosci. 2005. PMID: 15634780 Free PMC article.
-
PKCε promotes cardiac mitochondrial and metabolic adaptation to chronic hypobaric hypoxia by GSK3β inhibition.J Cell Physiol. 2011 Sep;226(9):2457-68. doi: 10.1002/jcp.22592. J Cell Physiol. 2011. PMID: 21660969 Free PMC article.
References
-
- Nicholls D G, Locke R M. Physiol Rev. 1984;64:1–64. - PubMed
-
- Cannon B, Nedergaard J. Essays Biochem. 1985;20:110–164. - PubMed
-
- Klingenberg M. Trends Biochem Sci. 1990;15:108–112. - PubMed
-
- Klingenberg M, Huang S G. Biochim Biophys Acta. 1999;1415:269–271. - PubMed
-
- Muzzin P, Boss O, Giacobino J P. J Bioenerg Biomembr. 1999;31:467–473. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

