Entry of human parechovirus 1
- PMID: 11160695
- PMCID: PMC115142
- DOI: 10.1128/JVI.75.4.1958-1967.2001
Entry of human parechovirus 1
Abstract
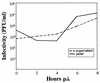
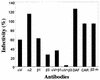
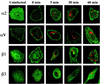
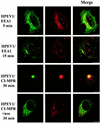
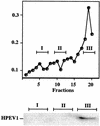
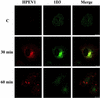
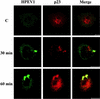
Human parechovirus 1 (HPEV-1) is a prototype member of parechoviruses, a recently established picornavirus genus. Although there is preliminary evidence that HPEV-1 recognizes alpha(V) integrins as cellular receptors, our understanding of early events during HPEV-1 infection is still very limited. The aim of this study was to clarify the entry mechanisms of HPEV-1, including the attachment of the virus onto the host cell surface and subsequent internalization. In blocking experiments with monoclonal antibodies against different receptor candidates, antibodies against alpha(V) and beta(3) integrin subunits, in particular in combination, appeared to be the most efficient ones in preventing the HPEV-1 infection. To find out whether HPEV-1 uses clathrin-coated vesicles or other routes for the entry into the host cell, we carried out double-labeling experiments of virus-infected cells with anti-HPEV-1 antibodies and antibodies against known markers of the clathrin and the caveolin routes. At the early phase of infection (5 min postinfection [p.i.]) HPEV-1 colocalized with EEA1 (early endosomes), and later, after 30 min p.i., it colocalized with mannose-6-phosphate receptor (late endosomes), whereas no colocalization with caveolin-1 was observed. The data indicate that HPEV-1 utilizes the clathrin-dependent endocytic pathway for entry into the host cells. Interestingly, endocytosed HPEV-1 capsid proteins were observed in the endoplasmic reticulum and cis-Golgi network 30 to 60 min p.i. Depolymerization of microtubules with nocodazole inhibited translocation of the virus to the late endosomes but did not block HPEV-1 replication, suggesting that the RNA genome may be released early during the entry process.
Figures

Similar articles
-
Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway.J Virol. 2004 Oct;78(19):10543-55. doi: 10.1128/JVI.78.19.10543-10555.2004. J Virol. 2004. PMID: 15367621 Free PMC article.
-
Analysis of foot-and-mouth disease virus internalization events in cultured cells.J Virol. 2005 Jul;79(13):8506-18. doi: 10.1128/JVI.79.13.8506-8518.2005. J Virol. 2005. PMID: 15956593 Free PMC article.
-
Clathrin- and caveolin-independent entry of human papillomavirus type 16--involvement of tetraspanin-enriched microdomains (TEMs).PLoS One. 2008 Oct 2;3(10):e3313. doi: 10.1371/journal.pone.0003313. PLoS One. 2008. PMID: 18836553 Free PMC article.
-
Caveosomes and endocytosis of lipid rafts.J Cell Sci. 2003 Dec 1;116(Pt 23):4707-14. doi: 10.1242/jcs.00840. J Cell Sci. 2003. PMID: 14600257 Review.
-
Cargo recognition during clathrin-mediated endocytosis: a team effort.Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
-
Molecular mechanism of alpha2beta1 integrin interaction with human echovirus 1.EMBO J. 2010 Jan 6;29(1):196-208. doi: 10.1038/emboj.2009.326. Epub 2009 Nov 19. EMBO J. 2010. PMID: 19927126 Free PMC article.
-
Integrin alpha v beta 6 is an RGD-dependent receptor for coxsackievirus A9.J Virol. 2004 Jul;78(13):6967-73. doi: 10.1128/JVI.78.13.6967-6973.2004. J Virol. 2004. PMID: 15194773 Free PMC article.
-
Endocytosis of integrin-binding human picornaviruses.Adv Virol. 2012;2012:547530. doi: 10.1155/2012/547530. Epub 2012 Nov 27. Adv Virol. 2012. PMID: 23227048 Free PMC article.
-
Arginine-glycine-aspartic acid motif is critical for human parechovirus 1 entry.J Virol. 2001 Oct;75(20):10000-4. doi: 10.1128/JVI.75.20.10000-10004.2001. J Virol. 2001. PMID: 11559835 Free PMC article.
-
Calpain 1 and 2 are required for RNA replication of echovirus 1.J Virol. 2008 Feb;82(3):1581-90. doi: 10.1128/JVI.01375-07. Epub 2007 Nov 21. J Virol. 2008. PMID: 18032503 Free PMC article.
References
-
- Banting G, Maile R, Roquemore E P. The steady state distribution of humTGN46 is not significantly altered in cells defective in clathrin-mediated endocytosis. J Cell Sci. 1998;111:3451–3458. - PubMed
-
- Bergelson J M, Shepley M P, Chan B M, Hemler M E, Finberg R W. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science. 1992;27:1718–1720. - PubMed
-
- Bergelson J M, Cunnigham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

