Quantitative relationship between transmitter release and calcium current at the calyx of held synapse
- PMID: 11160426
- PMCID: PMC6763832
- DOI: 10.1523/JNEUROSCI.21-02-00462.2001
Quantitative relationship between transmitter release and calcium current at the calyx of held synapse
Abstract
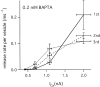
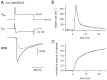
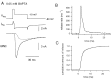
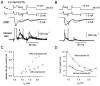
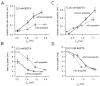
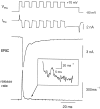
A newly developed deconvolution method (Neher and Sakaba, 2001) allowed us to resolve the time course of neurotransmitter release at the calyx of Held synapse and to quantify some basic aspects of transmitter release. First, we identified a readily releasable pool (RRP) of synaptic vesicles. We found that the size of the RRP, when tested with trains of strong stimuli, was constant regardless of the exact stimulus patterns, if stimuli were confined to a time interval of approximately 60 msec. For longer-lasting stimulus patterns, recruitment of new vesicles to the RRP made a substantial contribution to the total release. Second, the cooperativity of transmitter release as a function of Ca(2+) current was estimated to be 3-4, which confirmed previous results (Borst and Sakmann, 1999; Wu et al., 1999). Third, an initial small Ca(2+) influx increased the efficiency of Ca(2+) currents in subsequent transmitter release. This type of facilitation was blocked by a high concentration of EGTA (0.5 mm). Fourth, the release rates of synaptic vesicles at this synapse turned out to be heterogeneous: once a highly Ca(2+)-sensitive population of vesicles was consumed, the remaining vesicles released at lower rates. These components of release were more clearly separated in the presence of 0.5 mm EGTA, which prevented the buildup of residual Ca(2+). Conversely, raising the extracellular Ca(2+) concentration facilitated the slower population such that its release characteristics became more similar to those of the faster population under standard conditions. Heterogeneous release probabilities are expected to support the maintenance of synaptic transmission during high-frequency stimulation.
Figures
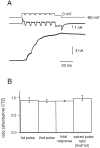
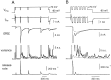
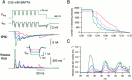
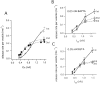

Similar articles
-
Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of held.J Neurosci. 2001 Jan 15;21(2):444-61. doi: 10.1523/JNEUROSCI.21-02-00444.2001. J Neurosci. 2001. PMID: 11160425 Free PMC article.
-
Presynaptic Ca2+ requirements and developmental regulation of posttetanic potentiation at the calyx of Held.J Neurosci. 2005 May 25;25(21):5127-37. doi: 10.1523/JNEUROSCI.1295-05.2005. J Neurosci. 2005. PMID: 15917453 Free PMC article.
-
Estimating transmitter release rates from postsynaptic current fluctuations.J Neurosci. 2001 Dec 15;21(24):9638-54. doi: 10.1523/JNEUROSCI.21-24-09638.2001. J Neurosci. 2001. PMID: 11739574 Free PMC article.
-
Kinetics of transmitter release at the calyx of Held synapse.Proc Jpn Acad Ser B Phys Biol Sci. 2018;94(3):139-152. doi: 10.2183/pjab.94.010. Proc Jpn Acad Ser B Phys Biol Sci. 2018. PMID: 29526973 Free PMC article. Review.
-
The calyx of Held.Cell Tissue Res. 2006 Nov;326(2):311-37. doi: 10.1007/s00441-006-0272-7. Epub 2006 Aug 8. Cell Tissue Res. 2006. PMID: 16896951 Review.
Cited by
-
A comparison between exocytic control mechanisms in adrenal chromaffin cells and a glutamatergic synapse.Pflugers Arch. 2006 Dec;453(3):261-8. doi: 10.1007/s00424-006-0143-9. Epub 2006 Oct 3. Pflugers Arch. 2006. PMID: 17016737 Review.
-
Genetic analysis of synaptotagmin 2 in spontaneous and Ca2+-triggered neurotransmitter release.EMBO J. 2006 May 17;25(10):2039-50. doi: 10.1038/sj.emboj.7601103. Epub 2006 Apr 27. EMBO J. 2006. PMID: 16642042 Free PMC article.
-
Separation of presynaptic and postsynaptic contributions to depression by covariance analysis of successive EPSCs at the calyx of Held synapse.J Neurosci. 2002 Feb 1;22(3):728-39. doi: 10.1523/JNEUROSCI.22-03-00728.2002. J Neurosci. 2002. PMID: 11826102 Free PMC article.
-
Calcium cooperativity of exocytosis as a measure of Ca²+ channel domain overlap.Brain Res. 2011 Jun 29;1398:126-38. doi: 10.1016/j.brainres.2011.05.011. Epub 2011 May 12. Brain Res. 2011. PMID: 21621748 Free PMC article. Review.
-
RIM-Binding Protein 2 Organizes Ca2+ Channel Topography and Regulates Release Probability and Vesicle Replenishment at a Fast Central Synapse.J Neurosci. 2021 Sep 15;41(37):7742-7767. doi: 10.1523/JNEUROSCI.0586-21.2021. Epub 2021 Aug 5. J Neurosci. 2021. PMID: 34353898 Free PMC article.
References
-
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

