Proteins associated with the promyelocytic leukemia gene product (PML)-containing nuclear body move to the nucleolus upon inhibition of proteasome-dependent protein degradation
- PMID: 11158586
- PMCID: PMC14700
- DOI: 10.1073/pnas.98.3.1012
Proteins associated with the promyelocytic leukemia gene product (PML)-containing nuclear body move to the nucleolus upon inhibition of proteasome-dependent protein degradation
Abstract
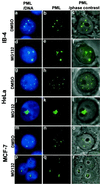
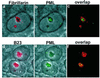
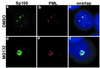
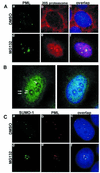
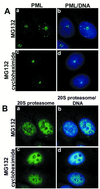
Several recent findings have indicated that the promyelocytic leukemia gene product (PML) oncogenic domains (PODs) are involved in proteasome-mediated degradation of ubiquitinated proteins. We wanted to examine the intracellular distribution of PML protein in the presence of a proteasome inhibitor. We used high-resolution microscopy to study the distribution of PML protein and other POD-associated proteins along with the proteasomes themselves under normal conditions and in cells treated with the proteasome inhibitor, MG132. Inhibition of the proteasomes in MCF-7, HeLa, and IB-4 cell lines resulted in a radical redistribution of the POD-associated proteins PML, Sp100, and SUMO-1. After 6-10 h of MG132 treatment, PML, Sp100, and SUMO-1 were no longer detectable in the PODs and accumulated mainly in the nucleolus. Moreover, MG132 treatment changed the cellular distribution of the proteasomes. Interestingly, this included the accumulation in euchromatin areas of the nucleus and within the nucleoli. Several non-POD-associated proteins did not change their cellular distribution under the same conditions. The accumulation of POD-associated proteins and proteasomes in the nucleoli of MG132-treated cells indicates that these proteins may target the nucleoli under normal conditions and that the nucleolus may have a function in the regulation of proteasomal protein degradation.
Figures
Similar articles
-
Interferon gamma regulates accumulation of the proteasome activator PA28 and immunoproteasomes at nuclear PML bodies.J Cell Sci. 2001 Jan;114(Pt 1):29-36. doi: 10.1242/jcs.114.1.29. J Cell Sci. 2001. PMID: 11112687
-
Proteasome-dependent processing of nuclear proteins is correlated with their subnuclear localization.J Struct Biol. 2002 Oct-Dec;140(1-3):189-99. doi: 10.1016/s1047-8477(02)00527-0. J Struct Biol. 2002. PMID: 12490167
-
Proteasome-independent disruption of PML oncogenic domains (PODs), but not covalent modification by SUMO-1, is required for human cytomegalovirus immediate-early protein IE1 to inhibit PML-mediated transcriptional repression.J Virol. 2001 Nov;75(22):10683-95. doi: 10.1128/JVI.75.22.10683-10695.2001. J Virol. 2001. PMID: 11602710 Free PMC article.
-
PML and COP1--two proteins with much in common.Trends Biochem Sci. 2001 Jan;26(1):18-20. doi: 10.1016/s0968-0004(00)01732-1. Trends Biochem Sci. 2001. PMID: 11165511 Review.
-
Intracellular localization of proteasomes.Int J Biochem Cell Biol. 2003 May;35(5):579-89. doi: 10.1016/s1357-2725(02)00380-1. Int J Biochem Cell Biol. 2003. PMID: 12672451 Review.
Cited by
-
The p97-UBXN1 complex regulates aggresome formation.J Cell Sci. 2021 Apr 1;134(7):jcs254201. doi: 10.1242/jcs.254201. Epub 2021 Apr 15. J Cell Sci. 2021. PMID: 33712450 Free PMC article.
-
The tumor suppressor SHIP1 colocalizes in nucleolar cavities with p53 and components of PML nuclear bodies.Nucleus. 2015;6(2):154-64. doi: 10.1080/19491034.2015.1022701. Epub 2015 Feb 27. Nucleus. 2015. PMID: 25723258 Free PMC article.
-
Evidence for ubiquitin-regulated nuclear and subnuclear trafficking among Paramyxovirinae matrix proteins.PLoS Pathog. 2015 Mar 17;11(3):e1004739. doi: 10.1371/journal.ppat.1004739. eCollection 2015 Mar. PLoS Pathog. 2015. PMID: 25782006 Free PMC article.
-
Epigenetics in the formation of pathological aggregates in amyotrophic lateral sclerosis.Front Mol Neurosci. 2024 Sep 3;17:1417961. doi: 10.3389/fnmol.2024.1417961. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39290830 Free PMC article. Review.
-
PML Bodies in Mitosis.Cells. 2019 Aug 14;8(8):893. doi: 10.3390/cells8080893. Cells. 2019. PMID: 31416160 Free PMC article. Review.
References
-
- Seeler J S, Dejean A. Curr Opin Genet Dev. 1999;9:362–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

