Comparison of DNA sequencing and a line probe assay for detection of human immunodeficiency virus type 1 drug resistance mutations in patients failing highly active antiretroviral therapy
- PMID: 11158089
- PMCID: PMC87758
- DOI: 10.1128/JCM.39.2.454-459.2001
Comparison of DNA sequencing and a line probe assay for detection of human immunodeficiency virus type 1 drug resistance mutations in patients failing highly active antiretroviral therapy
Abstract
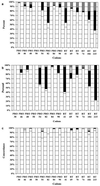
The resistance of human immunodeficiency virus type 1 (HIV-1) to drugs is a major cause of antiretroviral treatment failure. We have compared direct sequencing to a line probe assay (LiPA) for the detection of drug resistance-related mutations in 197 clinical samples, and we have investigated the sequential appearance of mutations under drug pressure. For 26 patients with virological failure despite the use of two nucleoside analogues and one protease inhibitor (indinavir [n = 6], ritonavir [n = 10], and saquinavir [n = 10]), genotypic resistance assays were carried out retrospectively every 3 months for up to 2 years by using direct sequencing (TruGene; Visible Genetics) and a LiPA for detection of mutations in the reverse transcriptase (INNO-LiPA HIV-1 RT; Innogenetics) and the protease (INNO-LiPA HIV Protease, prototype version; Innogenetics) genes. Comparison of the results from both assays found rare major discrepancies (<1% of codons analyzed). INNO-LiPA detected more wild-type-mutant mixtures than sequencing but suffered from a high rate of codon hybridization failures for the reverse transcriptase. LiPA detected earlier and more frequently than sequencing the transient mixed virus population that contained I84V, which appears before V82A in the protease sequence. Mutations M461, G48V, and L90M were often transient and drug pressure related. In conclusion, direct sequencing and LiPAs give concordant results for most clinical isolates. LiPAs are more sensitive for the detection of mixed virus populations. Mutation I84V appears in minor populations in the early steps of the pathways of resistance to indinavir and ritonavir. The fact that some mutations can be found only transiently and in minor virus populations highlights the importance of a low detection limit for resistance assays.
Figures
Similar articles
-
Comparative performance evaluation of the HIV-1 LiPA protease and reverse transcriptase resistance assay on clinical isolates.J Clin Virol. 2005 Dec;34(4):268-71. doi: 10.1016/j.jcv.2005.01.011. J Clin Virol. 2005. PMID: 16286050
-
Absence of resistance mutations in antiretroviral-naive patients treated with ritonavir-boosted saquinavir.Antivir Ther. 2006;11(5):631-5. Antivir Ther. 2006. PMID: 16964832 Clinical Trial.
-
Longitudinal use of a line probe assay for human immunodeficiency virus type 1 protease predicts phenotypic resistance and clinical progression in patients failing highly active antiretroviral therapy.Antimicrob Agents Chemother. 2002 Jun;46(6):1928-33. doi: 10.1128/AAC.46.6.1928-1933.2002. Antimicrob Agents Chemother. 2002. PMID: 12019110 Free PMC article.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Rational approaches to resistance: using saquinavir.AIDS. 1996 Nov;10 Suppl 1:S15-9. AIDS. 1996. PMID: 8970671 Review.
Cited by
-
Differential maintenance of the M184V substitution in the reverse transcriptase of human immunodeficiency virus type 1 by various nucleoside antiretroviral agents in tissue culture.Antimicrob Agents Chemother. 2004 Nov;48(11):4189-94. doi: 10.1128/AAC.48.11.4189-4194.2004. Antimicrob Agents Chemother. 2004. PMID: 15504840 Free PMC article.
-
Fluorescent dye terminator sequencing methods for quantitative determination of replication fitness of human immunodeficiency virus type 1 containing the codon 74 and 184 mutations in reverse transcriptase.J Clin Microbiol. 2003 Jul;41(7):3306-11. doi: 10.1128/JCM.41.7.3306-3311.2003. J Clin Microbiol. 2003. PMID: 12843079 Free PMC article.
-
Mutagenically separated PCR assay for rapid detection of M41L and K70R zidovudine resistance mutations in CRF01_AE (subtype E) human immunodeficiency virus type 1.Antimicrob Agents Chemother. 2002 Dec;46(12):3861-8. doi: 10.1128/AAC.46.12.3861-3868.2002. Antimicrob Agents Chemother. 2002. PMID: 12435689 Free PMC article.
-
Comparison between sequence analysis and a line probe assay for testing genotypic resistance of human immunodeficiency virus type 1 to antiretroviral drugs.J Clin Microbiol. 2005 Aug;43(8):4186-8. doi: 10.1128/JCM.43.8.4186-4188.2005. J Clin Microbiol. 2005. PMID: 16081972 Free PMC article.
-
A Guide to HIV-1 Reverse Transcriptase and Protease Sequencing for Drug Resistance Studies.HIV Seq Compend. 2001;2001:1-51. HIV Seq Compend. 2001. PMID: 22324021 Free PMC article. No abstract available.
References
-
- Collier A, Coombs R W, Schoenfeld D A, Bassett R L, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe V J, Friedman H M, Merigan T C, Reichman R C, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N Engl J Med. 1996;334:1011–1017. - PubMed
-
- Condra J H, Holder D J, Schleif W A, Blahy O M, Danovich R M, Gabryelski L J, Graham D J, Laird D, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, Yang T, Chodakewitz J A, Deutsch P J, Leavitt R Y, Massari F E, Mellors J W, Squires K E, Steigbigel R T, Teppler H, Emini E A. Genetic correlates of in vivo viral resistance to indinavir, a human immunodeficiency virus type 1 inhibitor. J Virol. 1996;70:8270–8276. - PMC - PubMed
-
- Condra J H, Schleif W A, Blahy O M, Gabryelski L J, Graham D J, Quintero J C, Rhodes A, Robbins H L, Roth E, Shivaprakash M, Titus D, Yang T, Teppler H, Squires K E, Deutsch P J, Emini E A. In vivo emergence of HIV-1 variants resistant to multiple protease inhibitors. Nature. 1995;374:569–571. - PubMed
-
- Descamps D, Calvez V, Collin G, Cécille A, Apetrei C, Damond F, Katlama C, Matheron S, Huraux J M, Brun-Vézinet F. Line probe assay for detection of human immunodeficiency virus type 1 mutations conferring resistance to nucleoside inhibitors of reverse transcriptase: comparison with sequence analysis. J Clin Microbiol. 1998;36:2143–2145. - PMC - PubMed
-
- Devereux H L, Youle M, Johnson M A, Loveday C. Rapid decline in detectability of HIV-1 drug resistance mutations after stopping therapy. AIDS. 1999;13:123–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

