DIABLO promotes apoptosis by removing MIHA/XIAP from processed caspase 9
- PMID: 11157976
- PMCID: PMC2195997
- DOI: 10.1083/jcb.152.3.483
DIABLO promotes apoptosis by removing MIHA/XIAP from processed caspase 9
Abstract
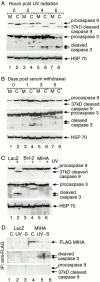
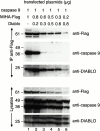
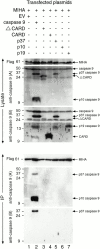
MIHA is an inhibitor of apoptosis protein (IAP) that can inhibit cell death by direct interaction with caspases, the effector proteases of apoptosis. DIABLO is a mammalian protein that can bind to IAPs and antagonize their antiapoptotic effect, a function analogous to that of the proapoptotic Drosophila molecules, Grim, Reaper, and HID. Here, we show that after UV radiation, MIHA prevented apoptosis by inhibiting caspase 9 and caspase 3 activation. Unlike Bcl-2, MIHA functioned after release of cytochrome c and DIABLO from the mitochondria and was able to bind to both processed caspase 9 and processed caspase 3 to prevent feedback activation of their zymogen forms. Once released into the cytosol, DIABLO bound to MIHA and disrupted its association with processed caspase 9, thereby allowing caspase 9 to activate caspase 3, resulting in apoptosis.
Figures


Similar articles
-
Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2.EMBO J. 2001 Dec 3;20(23):6627-36. doi: 10.1093/emboj/20.23.6627. EMBO J. 2001. PMID: 11726499 Free PMC article.
-
Molecular determinants of the caspase-promoting activity of Smac/DIABLO and its role in the death receptor pathway.J Biol Chem. 2000 Nov 17;275(46):36152-7. doi: 10.1074/jbc.C000533200. J Biol Chem. 2000. PMID: 10950947
-
Effects of antioxidants and caspase-3 inhibitor on the phenylethyl isothiocyanate-induced apoptotic signaling pathways in human PLC/PRF/5 cells.Eur J Pharmacol. 2005 Aug 22;518(2-3):96-106. doi: 10.1016/j.ejphar.2005.06.021. Eur J Pharmacol. 2005. PMID: 16054126
-
Mammalian mitochondrial IAP binding proteins.Biochem Biophys Res Commun. 2003 May 9;304(3):499-504. doi: 10.1016/s0006-291x(03)00622-3. Biochem Biophys Res Commun. 2003. PMID: 12729584 Review.
-
Cell death regulation by the mammalian IAP antagonist Diablo/Smac.Apoptosis. 2002 Apr;7(2):163-6. doi: 10.1023/a:1014318615955. Apoptosis. 2002. PMID: 11865200 Review.
Cited by
-
Mathematical modeling identifies inhibitors of apoptosis as mediators of positive feedback and bistability.PLoS Comput Biol. 2006 Sep 15;2(9):e120. doi: 10.1371/journal.pcbi.0020120. Epub 2006 Jul 28. PLoS Comput Biol. 2006. PMID: 16978046 Free PMC article.
-
Prenatal exposure to chromium induces early reproductive senescence by increasing germ cell apoptosis and advancing germ cell cyst breakdown in the F1 offspring.Dev Biol. 2014 Apr 1;388(1):22-34. doi: 10.1016/j.ydbio.2014.02.003. Epub 2014 Feb 12. Dev Biol. 2014. PMID: 24530425 Free PMC article.
-
In Vitro Pro-apoptotic and Anti-migratory Effects of Ficus deltoidea L. Plant Extracts on the Human Prostate Cancer Cell Lines PC3.Front Pharmacol. 2017 Dec 12;8:895. doi: 10.3389/fphar.2017.00895. eCollection 2017. Front Pharmacol. 2017. PMID: 29326585 Free PMC article.
-
Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2.EMBO J. 2001 Dec 3;20(23):6627-36. doi: 10.1093/emboj/20.23.6627. EMBO J. 2001. PMID: 11726499 Free PMC article.
-
Jafrac2 is an IAP antagonist that promotes cell death by liberating Dronc from DIAP1.EMBO J. 2002 Oct 1;21(19):5118-29. doi: 10.1093/emboj/cdf530. EMBO J. 2002. PMID: 12356728 Free PMC article.
References
-
- Day C.L., Dupont C., Lackmann M., Vaux D.L., Hinds M.G. Solution structure and mutagenesis of the caspase recruitment domain (CARD) from Apaf-1. Cell Death Differ. 1999;6:1125–1132. - PubMed
-
- Deveraux Q.L., Takahashi R., Salvesen G.S., Reed J.C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

