The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice
- PMID: 11150314
- PMCID: PMC6762458
- DOI: 10.1523/JNEUROSCI.21-01-00010.2001
The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice
Abstract
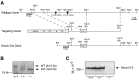
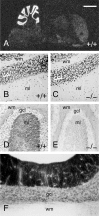
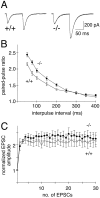
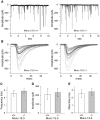
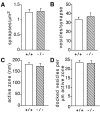
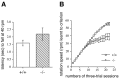
Munc13 proteins form a family of three, primarily brain-specific phorbol ester receptors (Munc13-1/2/3) in mammals. Munc13-1 is a component of presynaptic active zones in which it acts as an essential synaptic vesicle priming protein. In contrast to Munc13-1, which is present in most neurons throughout the rat and mouse CNS, Munc13-3 is almost exclusively expressed in the cerebellum. Munc13-3 mRNA is present in granule and Purkinje cells but absent from glia cells. Munc13-3 protein is localized to the synaptic neuropil of the cerebellar molecular layer but is not found in Purkinje cell dendrites, suggesting that Munc13-3, like Munc13-1, is a presynaptic protein at parallel fiber-Purkinje cell synapses. To examine the role of Munc13-3 in cerebellar physiology, we generated Munc13-3-deficient mutant mice. Munc13-3 deletion mutants exhibit increased paired-pulse facilitation at parallel fiber-Purkinje cell synapses. In addition, mutant mice display normal spontaneous motor activity but have an impaired ability to learn complex motor tasks. Our data demonstrate that Munc13-3 regulates synaptic transmission at parallel fiber-Purkinje cell synapses. We propose that Munc13-3 acts at a similar step of the synaptic vesicle cycle as does Munc13-1, albeit with less efficiency. In view of the present data and the well established vesicle priming function of Munc13-1, it is likely that Munc13-3-loss leads to a reduction in release probability at parallel fiber-Purkinje cell synapses by interfering with vesicle priming. This, in turn, would lead to increases in paired-pulse facilitation and could contribute to the observed deficit in motor learning.
Figures
Similar articles
-
Fast cerebellar reflex circuitry requires synaptic vesicle priming by munc13-3.Cerebellum. 2015 Jun;14(3):264-83. doi: 10.1007/s12311-015-0645-0. Cerebellum. 2015. PMID: 25617111 Free PMC article.
-
Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure.J Neurosci. 2002 Feb 15;22(4):1316-27. doi: 10.1523/JNEUROSCI.22-04-01316.2002. J Neurosci. 2002. PMID: 11850459 Free PMC article.
-
Munc13-3 superprimes synaptic vesicles at granule cell-to-basket cell synapses in the mouse cerebellum.J Neurosci. 2014 Oct 29;34(44):14687-96. doi: 10.1523/JNEUROSCI.2060-14.2014. J Neurosci. 2014. PMID: 25355221 Free PMC article.
-
Functions of glutamate transporters in cerebellar Purkinje cell synapses.Acta Physiol (Oxf). 2009 Sep;197(1):1-12. doi: 10.1111/j.1748-1716.2009.02019.x. Epub 2009 Jul 6. Acta Physiol (Oxf). 2009. PMID: 19583702 Review.
-
mGluR1 signaling in cerebellar Purkinje cells: Subcellular organization and involvement in cerebellar function and disease.Neuropharmacology. 2021 Aug 15;194:108629. doi: 10.1016/j.neuropharm.2021.108629. Epub 2021 Jun 4. Neuropharmacology. 2021. PMID: 34089728 Review.
Cited by
-
Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming.Proc Natl Acad Sci U S A. 2002 Jun 25;99(13):9037-42. doi: 10.1073/pnas.122623799. Epub 2002 Jun 17. Proc Natl Acad Sci U S A. 2002. PMID: 12070347 Free PMC article.
-
Bidirectional regulation of Munc13-3 protein expression by age and dark rearing during the critical period in mouse visual cortex.Neuroscience. 2007 Dec 12;150(3):603-8. doi: 10.1016/j.neuroscience.2007.09.053. Epub 2007 Sep 29. Neuroscience. 2007. PMID: 17997229 Free PMC article.
-
A fluorescent nanosensor paint detects dopamine release at axonal varicosities with high spatiotemporal resolution.Proc Natl Acad Sci U S A. 2022 May 31;119(22):e2202842119. doi: 10.1073/pnas.2202842119. Epub 2022 May 25. Proc Natl Acad Sci U S A. 2022. PMID: 35613050 Free PMC article.
-
Tumor Necrosis Factor Alpha and Insulin-Like Growth Factor 1 Induced Modifications of the Gene Expression Kinetics of Differentiating Skeletal Muscle Cells.PLoS One. 2015 Oct 8;10(10):e0139520. doi: 10.1371/journal.pone.0139520. eCollection 2015. PLoS One. 2015. PMID: 26447881 Free PMC article.
-
Loss of MUNC13-1 function causes microcephaly, cortical hyperexcitability, and fatal myasthenia.Neurol Genet. 2016 Sep 8;2(5):e105. doi: 10.1212/NXG.0000000000000105. eCollection 2016 Oct. Neurol Genet. 2016. PMID: 27648472 Free PMC article.
References
-
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999b;400:457–461. - PubMed
-
- Betz A, Benseler F, Okamoto M, Brose N. Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin. J Biol Chem. 1997;272:2520–2526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
