Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation
- PMID: 11134510
- PMCID: PMC14592
- DOI: 10.1073/pnas.98.1.343
Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation
Abstract
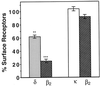
G-protein-coupled receptors (GPCRs) have recently joined the list of cell surface receptors that dimerize. Dimerization has been shown to alter the ligand-binding, signaling, and trafficking properties of these receptors. Recent studies have shown that GPCRs heterodimerize with closely related members, resulting in the modulation of their function. In this study, we have attempted to determine whether members of GPCR superfamilies that couple to different families of G-proteins can associate and form oligomers. We chose the beta2 adrenergic receptor that couples to stimulatory G-proteins and delta & kappa opioid receptors that couple to inhibitory G-proteins. beta2 and delta receptors undergo robust agonist-mediated endocytosis, whereas kappa receptors do not. We find that when coexpressed, beta2 receptors can form heteromeric complexes with both delta and kappa receptors. This heterooligomerization does not significantly alter the ligand binding or coupling properties of the receptors. However, it affects the trafficking properties of the receptors. For example, we find that delta receptors, when coexpressed with beta2 receptors, undergo isoproterenol-mediated endocytosis. Conversely, beta2 receptors in these cells undergo etorphine-mediated endocytosis. However, beta2 receptors, when coexpressed with kappa receptors, undergo neither opioid- nor isoproterenol-mediated endocytosis. Moreover, these cells exhibit a substantial decrease in the isoproterenol-induced phosphorylation of mitogen-activated protein kinases. Taken together, these results provide direct evidence of heteromerization of GPCRs that couple to different types of G-proteins, which results in the modulation of receptor trafficking and signal transduction.
Figures
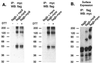
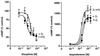

Similar articles
-
Differential effects of opioid and adrenergic agonists on proliferation in a cultured cell line.Cell Prolif. 1999 Aug;32(4):215-29. doi: 10.1046/j.1365-2184.1999.3240215.x. Cell Prolif. 1999. PMID: 10614711 Free PMC article.
-
Hetero-oligomerization between beta2- and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties.J Biol Chem. 2004 Jul 2;279(27):28756-65. doi: 10.1074/jbc.M313310200. Epub 2004 Apr 27. J Biol Chem. 2004. PMID: 15123695
-
Opioid receptor endocytosis and activation of MAP kinase pathway.Brain Res Mol Brain Res. 2000 Mar 29;76(2):220-8. doi: 10.1016/s0169-328x(00)00002-4. Brain Res Mol Brain Res. 2000. PMID: 10762697
-
Homo- and hetero-oligomerization of β2-adrenergic receptor in receptor trafficking, signaling pathways and receptor pharmacology.Cell Signal. 2014 Oct;26(10):2259-65. doi: 10.1016/j.cellsig.2014.06.016. Epub 2014 Jul 15. Cell Signal. 2014. PMID: 25049076 Review.
-
Opioid receptor trafficking and signaling: what happens after opioid receptor activation?Cell Mol Neurobiol. 2012 Mar;32(2):167-84. doi: 10.1007/s10571-011-9755-5. Epub 2011 Sep 25. Cell Mol Neurobiol. 2012. PMID: 21947865 Review.
Cited by
-
Interclass GPCR heteromerization affects localization and trafficking.Sci Signal. 2020 Oct 20;13(654):eaaw3122. doi: 10.1126/scisignal.aaw3122. Sci Signal. 2020. PMID: 33082287 Free PMC article.
-
Complement component 5a receptor oligomerization and homologous receptor down-regulation.J Biol Chem. 2008 Nov 7;283(45):31038-46. doi: 10.1074/jbc.M805260200. Epub 2008 Sep 4. J Biol Chem. 2008. PMID: 18772131 Free PMC article.
-
Adenosine A1 receptors heterodimerize with β1- and β2-adrenergic receptors creating novel receptor complexes with altered G protein coupling and signaling.Cell Signal. 2013 Apr;25(4):736-42. doi: 10.1016/j.cellsig.2012.12.022. Epub 2013 Jan 3. Cell Signal. 2013. PMID: 23291003 Free PMC article.
-
Interaction and regulatory functions of μ- and δ-opioid receptors in nociceptive afferent neurons.Neurosci Bull. 2012 Apr;28(2):121-30. doi: 10.1007/s12264-012-1206-x. Neurosci Bull. 2012. PMID: 22466123 Free PMC article. Review.
-
Regulation of neuronal PLCgamma by chronic morphine.Brain Res. 2007 Jul 2;1156:9-20. doi: 10.1016/j.brainres.2007.04.059. Epub 2007 May 4. Brain Res. 2007. PMID: 17524370 Free PMC article.
References
-
- Gether U. Endocr Rev. 2000;21:90–113. - PubMed
-
- Hebert I E, Bouvier M. Biochem Cell Biol. 1998;76:1–11. - PubMed
-
- Marshall F H, Jones K A, Kaupmann K, Bettler B. Trends Pharmacol Sci. 1999;20:396–399. - PubMed
-
- Jones K A, Borowsky B, Tamm J A, Craig D A, Durkin M M, Dai M, Yao W-J, Johnson M, Gunwaldsen C, Huang L-Y, et al. Nature (London) 1998;396:674–679. - PubMed
-
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, et al. Nature (London) 1998;396:683–687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

