Adenovirus protein V induces redistribution of nucleolin and B23 from nucleolus to cytoplasm
- PMID: 11134316
- PMCID: PMC113999
- DOI: 10.1128/JVI.75.2.1031-1038.2001
Adenovirus protein V induces redistribution of nucleolin and B23 from nucleolus to cytoplasm
Abstract
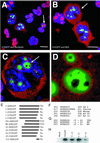
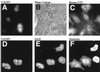
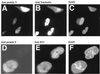
Adenovirus infection inhibits synthesis and processing of rRNA and redistributes nucleolar antigens. Adenovirus protein V associates with nucleoli in infected cells. This study delineates regions of protein V independently capable of nucleolar targeting. Also, evidence is presented that protein V has the unique property of relocating nucleolin and B23 to the cytoplasm when transiently expressed on its own in uninfected cells. Point mutation analysis indicates a role for the C terminus of protein V in the redirection of nucleolin and B23 to the cytoplasm. This is the first time an adenovirus protein has been shown to have a direct effect on nucleolar antigens in isolation from viral infection. Moreover, adenovirus protein V is the first protein demonstrated to be capable of redirecting nucleolin and B23 to the cytoplasm.
Figures

Similar articles
-
Nucleolar localization of aprataxin is dependent on interaction with nucleolin and on active ribosomal DNA transcription.Hum Mol Genet. 2006 Jul 15;15(14):2239-49. doi: 10.1093/hmg/ddl149. Epub 2006 Jun 15. Hum Mol Genet. 2006. PMID: 16777843
-
Identification of nucleophosmin/B23, an acidic nucleolar protein, as a stimulatory factor for in vitro replication of adenovirus DNA complexed with viral basic core proteins.J Mol Biol. 2001 Aug 3;311(1):41-55. doi: 10.1006/jmbi.2001.4812. J Mol Biol. 2001. PMID: 11469856
-
A 110-kDa nuclear shuttle protein, nucleolin, specifically binds to adeno-associated virus type 2 (AAV-2) capsid.Virology. 1999 May 10;257(2):373-82. doi: 10.1006/viro.1999.9664. Virology. 1999. PMID: 10329548
-
The roles of nucleolin subcellular localization in cancer.Biochimie. 2015 Jun;113:78-85. doi: 10.1016/j.biochi.2015.03.023. Epub 2015 Apr 9. Biochimie. 2015. PMID: 25866190 Review.
-
Plant nucleolar DNA: Green light shed on the role of Nucleolin in genome organization.Nucleus. 2017 Jan 2;8(1):11-16. doi: 10.1080/19491034.2016.1236167. Epub 2016 Sep 20. Nucleus. 2017. PMID: 27644794 Free PMC article. Review.
Cited by
-
Nullbasic, a potent anti-HIV tat mutant, induces CRM1-dependent disruption of HIV rev trafficking.PLoS One. 2012;7(12):e51466. doi: 10.1371/journal.pone.0051466. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251541 Free PMC article.
-
Effect of hepatitis C virus (HCV) NS5B-nucleolin interaction on HCV replication with HCV subgenomic replicon.J Virol. 2006 Apr;80(7):3332-40. doi: 10.1128/JVI.80.7.3332-3340.2006. J Virol. 2006. PMID: 16537600 Free PMC article.
-
Nuclear remodelling during viral infections.Cell Microbiol. 2011 Jun;13(6):806-13. doi: 10.1111/j.1462-5822.2011.01596.x. Epub 2011 Apr 28. Cell Microbiol. 2011. PMID: 21501365 Free PMC article. Review.
-
Isolation and characterization of the DNA and protein binding activities of adenovirus core protein V.J Virol. 2014 Aug;88(16):9287-96. doi: 10.1128/JVI.00935-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899200 Free PMC article.
-
Host Subcellular Organelles: Targets of Viral Manipulation.Int J Mol Sci. 2024 Jan 29;25(3):1638. doi: 10.3390/ijms25031638. Int J Mol Sci. 2024. PMID: 38338917 Free PMC article. Review.
References
-
- Askham J M, Moncur P, Markham A F, Morrison E E. Regulation and function of the interaction between the APC tumour suppressor protein and EB1. Oncogene. 2000;19:1950–1958. - PubMed
-
- Borer R A, Lehner C F, Eppenberger H M, Nigg E A. Major nucleolar proteins shuttle between nucleolus and cytoplasm. Cell. 1989;56:379–390. - PubMed
-
- Chen C M, Chiang S Y, Yeh N H. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

