Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling
- PMID: 11134186
- PMCID: PMC198547
- DOI: 10.1172/JCI10741
Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling
Abstract
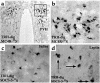
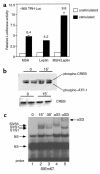
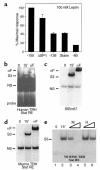
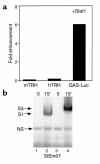
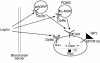
Starvation causes a rapid reduction in thyroid hormone levels in rodents. This adaptive response is caused by a reduction in thyrotropin-releasing hormone (TRH) expression that can be reversed by the administration of leptin. Here we examined hypothalamic signaling pathways engaged by leptin to upregulate TRH gene expression. As assessed by leptin-induced expression of suppressor of cytokine signaling-3 (SOCS-3) in fasted rats, TRH neurons in the paraventricular nucleus are activated directly by leptin. To a greater degree, they also contain melanocortin-4 receptors (MC4Rs), implying that leptin can act directly or indirectly by increasing the production of the MC4R ligand, alpha-melanocyte stimulating hormone (alpha-MSH), to regulate TRH expression. We further demonstrate that both pathways converge on the TRH promoter. The melanocortin system activates the TRH promoter through the phosphorylation and DNA binding of the cAMP response element binding protein (CREB), and leptin signaling directly regulates the TRH promoter through the phosphorylation of signal transducer and activator of transcription 3 (Stat3). Indeed, a novel Stat-response element in the TRH promoter is necessary for leptin's effect. Thus, the TRH promoter is an ideal target for further characterizing the integration of transcriptional pathways through which leptin acts.
Figures
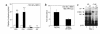


Similar articles
-
Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo.Endocrinology. 2004 May;145(5):2221-7. doi: 10.1210/en.2003-1312. Epub 2004 Feb 5. Endocrinology. 2004. PMID: 14764630
-
Intracerebroventricular administration of alpha-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus.Brain Res. 2002 Jul 26;945(1):50-9. doi: 10.1016/s0006-8993(02)02619-7. Brain Res. 2002. PMID: 12113951
-
Central administration of neuropeptide Y reduces alpha-melanocyte-stimulating hormone-induced cyclic adenosine 5'-monophosphate response element binding protein (CREB) phosphorylation in pro-thyrotropin-releasing hormone neurons and increases CREB phosphorylation in corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus.Endocrinology. 2003 Jan;144(1):281-91. doi: 10.1210/en.2002-220675. Endocrinology. 2003. PMID: 12488356
-
Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis.Peptides. 2006 Feb;27(2):310-25. doi: 10.1016/j.peptides.2005.01.033. Epub 2005 Nov 28. Peptides. 2006. PMID: 16310285 Review.
-
The TRH neuron: a hypothalamic integrator of energy metabolism.Prog Brain Res. 2006;153:209-35. doi: 10.1016/S0079-6123(06)53012-2. Prog Brain Res. 2006. PMID: 16876577 Review.
Cited by
-
AgRP and POMC neurons are hypophysiotropic and coordinately regulate multiple endocrine axes in a larval teleost.Cell Metab. 2012 Feb 8;15(2):256-64. doi: 10.1016/j.cmet.2011.12.014. Epub 2012 Jan 12. Cell Metab. 2012. PMID: 22245570 Free PMC article.
-
Melanocortin-4 Receptor PLC Activation Is Modulated by an Interaction with the Monocarboxylate Transporter 8.Int J Mol Sci. 2024 Jul 10;25(14):7565. doi: 10.3390/ijms25147565. Int J Mol Sci. 2024. PMID: 39062808 Free PMC article.
-
Effect of TSH on Non-Alcoholic Fatty Liver Disease (NAFLD) independent of obesity in children of predominantly Hispanic/Latino ancestry by causal mediation analysis.PLoS One. 2020 Jun 22;15(6):e0234985. doi: 10.1371/journal.pone.0234985. eCollection 2020. PLoS One. 2020. PMID: 32569304 Free PMC article.
-
Risk factors of subclinical hypothyroidism and the potential contribution to miscarriage: A review.Reprod Med Biol. 2020 Mar 18;19(3):232-242. doi: 10.1002/rmb2.12325. eCollection 2020 Jul. Reprod Med Biol. 2020. PMID: 32684822 Free PMC article. Review.
-
The metabolic actions of thyroid hormone and leptin: a mandatory interplay or not?Diabetologia. 2005 Apr;48(4):621-3. doi: 10.1007/s00125-005-1703-9. Epub 2005 Mar 12. Diabetologia. 2005. PMID: 15765220 No abstract available.
References
-
- Segerson TP, et al. Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science. 1987;238:78–80. - PubMed
-
- Blake NG, Eckland DJ, Foster OJ, Lightman SL. Inhibition of hypothalamic thyrotropin-releasing hormone messenger ribonucleic acid during food deprivation. Endocrinology. 1991;129:2714–2718. - PubMed
-
- Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. - PubMed
-
- Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

