Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation
- PMID: 11121077
- PMCID: PMC18994
- DOI: 10.1073/pnas.97.26.14772
Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation
Abstract
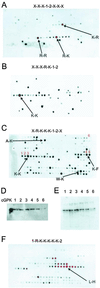
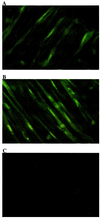
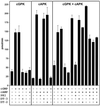
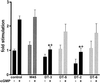
Arrays of octameric peptide libraries on cellulose paper were screened by using (32)P-autophosphorylated cGMP-dependent protein kinase Ialpha (cGPK) to identify peptide sequences with high binding affinity for cGPK. Iterative deconvolution of every amino acid position in the peptides identified the sequence LRK(5)H (W45) as having the highest binding affinity. Binding of W45 to cGPK resulted in selective inhibition of the kinase with K(i) values of 0.8 microM and 560 microM for cGPK and cAMP-dependent protein kinase (cAPK), respectively. Fusion of W45 to membrane translocation signals from HIV-1 tat protein (YGRKKRRQRRRPP-LRK(5)H, DT-2) or Drosophila Antennapedia homeo-domain (RQIKIWFQNRRMKWKK-LRK(5)H, DT-3) proved to be an efficient method for intracellular delivery of these highly charged peptides. Rapid translocation of the peptides into intact cerebral arteries was demonstrated by using fluorescein-labeled DT-2 and DT-3. The inhibitory potency of the fusion peptides was even greater than that for W45, with K(i) values of 12.5 nM and 25 nM for DT-2 and DT-3, respectively. Both peptides were still poor inhibitors of cAPK. Selective inhibition of cGPK by DT-2 or DT-3 in the presence of cAPK was demonstrated in vitro. In pressurized cerebral arteries, DT-2 and DT-3 substantially decreased NO-induced dilation. This study provides functional characterization of a class of selective cGPK inhibitor peptides in vascular smooth muscle and reveals a central role for cGPK in the modulation of vascular contractility.
Figures
Similar articles
-
Exploring the mechanisms of vascular smooth muscle tone with highly specific, membrane-permeable inhibitors of cyclic GMP-dependent protein kinase Ialpha.Pharmacol Ther. 2002 Feb-Mar;93(2-3):203-15. doi: 10.1016/s0163-7258(02)00189-4. Pharmacol Ther. 2002. PMID: 12191612 Review.
-
Inhibition of cGMP-dependent protein kinase by the cell-permeable peptide DT-2 reveals a novel mechanism of vasoregulation.Mol Pharmacol. 2004 May;65(5):1111-9. doi: 10.1124/mol.65.5.1111. Mol Pharmacol. 2004. PMID: 15102939
-
Delineation of selective cyclic GMP-dependent protein kinase Ialpha substrate and inhibitor peptides based on combinatorial peptide libraries on paper.Pharmacol Ther. 1999 May-Jun;82(2-3):373-87. doi: 10.1016/s0163-7258(98)00063-1. Pharmacol Ther. 1999. PMID: 10454213
-
Culture conditions influence uptake and intracellular localization of the membrane permeable cGMP-dependent protein kinase inhibitor DT-2.Front Biosci. 2005 May 1;10:1302-12. doi: 10.2741/1620. Front Biosci. 2005. PMID: 15769626
-
(D)-Amino acid analogues of DT-2 as highly selective and superior inhibitors of cGMP-dependent protein kinase Ialpha.Biochim Biophys Acta. 2010 Mar;1804(3):524-32. doi: 10.1016/j.bbapap.2009.12.004. Epub 2009 Dec 16. Biochim Biophys Acta. 2010. PMID: 20018259 Free PMC article.
Cited by
-
Sub-Nanomolar Sensitivity of Nitric Oxide Mediated Regulation of cGMP and Vasomotor Reactivity in Vascular Smooth Muscle.Front Pharmacol. 2012 Jul 12;3:130. doi: 10.3389/fphar.2012.00130. eCollection 2012. Front Pharmacol. 2012. PMID: 22807915 Free PMC article.
-
Probing noncovalent protein-ligand interactions of the cGMP-dependent protein kinase using electrospray ionization time of flight mass spectrometry.J Am Soc Mass Spectrom. 2004 Oct;15(10):1392-1399. doi: 10.1016/j.jasms.2004.06.015. J Am Soc Mass Spectrom. 2004. PMID: 15465351
-
Inhibition of the mitochondrial permeability transition by protein kinase A in rat liver mitochondria and hepatocytes.Biochem J. 2010 Nov 1;431(3):411-21. doi: 10.1042/BJ20091741. Biochem J. 2010. PMID: 20738255 Free PMC article.
-
Mode of action of cGMP-dependent protein kinase-specific inhibitors probed by photoaffinity cross-linking mass spectrometry.J Biol Chem. 2009 Jun 12;284(24):16354-16368. doi: 10.1074/jbc.M808521200. Epub 2009 Apr 15. J Biol Chem. 2009. PMID: 19369251 Free PMC article.
-
Application of microarrays in high-throughput enzymatic profiling.Mol Biotechnol. 2004 Nov;28(3):227-39. doi: 10.1385/MB:28:3:227. Mol Biotechnol. 2004. PMID: 15542923 Review.
References
-
- Lincoln T M, Komalavilas P, Boerth N J, Mac-Millan-Crow L A, Cornwell T L. Adv Pharmacol (San Diego) 1995;34:305–322. - PubMed
-
- Pfeifer A, Ruth P, Dostmann W, Sausbier M, Klatt P, Hofmann F. Rev Physiol Biochem Pharmacol. 1999;135:105–149. - PubMed
-
- Surks H K, Mochizuki N, Kasai Y, Georgescu S P, Tang K M, Ito M, Lincoln T M, Mendelsohn M E. Science. 1999;286:1583–1587. - PubMed
-
- Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang G-X, Allescher H D, Korth M, Wilm M, et al. Nature (London) 2000;404:197–201. - PubMed
-
- Pfeifer A, Aszodi A, Seidler U, Ruth P, Hofmann F, Fassler R. Science. 1996;274:2082–2086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

