Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument
- PMID: 11119602
- PMCID: PMC113926
- DOI: 10.1128/JVI.75.1.323-340.2001
Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument
Abstract
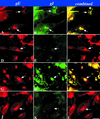
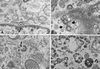
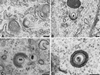
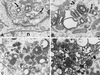
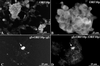
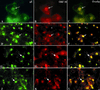
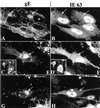
Varicella-zoster virus (VZV) is enveloped in the trans-Golgi network (TGN). Here we report that glycoprotein I (gI) is required within the TGN for VZV envelopment. Enveloping membranous TGN cisternae were microscopically identified in cells infected with intact VZV. These sacs curved around, and ultimately enclosed, nucleocapsids. Tegument coated the concave face of these sacs, which formed the viral envelope, but the convex surface was tegument-free. TGN cisternae of cells infected with VZV mutants lacking gI (gI(Delta)) or its C (gI(DeltaC))- or N-terminal (gI(DeltaN))-terminal domains were uniformly tegument coated and adhered to one another, forming bizarre membranous stacks. Viral envelopment was compromised, and no virions were delivered to post-Golgi structures. The TGN was not gI-immunoreactive in cells infected with the gI(Delta) or gI(DeltaN) mutants, but it was in cells infected with gI(DeltaC) (because the ectodomains of gI and gE interact). The presence in the TGN of gI lacking a C-terminal domain, therefore, was not sufficient to maintain enveloping cisternae. In cells infected with intact VZV or with gI(Delta), gI(DeltaN), or gI(DeltaC) mutants, ORF10p immunoreactivity was concentrated on the cytosolic face of TGN membranes, suggesting that it interacts with the cytosolic domains of glycoproteins. Because of the gE-gI interaction, cotransfected cells that expressed gE or gI were able to target truncated forms of the other to the TGN. Our data suggest that the C-terminal domain of gI is required to segregate viral and cellular proteins in enveloping TGN cisternae.
Figures


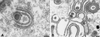

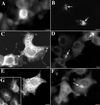
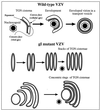
Similar articles
-
Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network.J Virol. 1994 Oct;68(10):6372-90. doi: 10.1128/JVI.68.10.6372-6390.1994. J Virol. 1994. PMID: 8083976 Free PMC article.
-
Intracellular transport of varicella-zoster glycoproteins.J Infect Dis. 1998 Nov;178 Suppl 1:S7-12. doi: 10.1086/514268. J Infect Dis. 1998. PMID: 9852965
-
Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network.J Biol Chem. 1998 May 29;273(22):13430-6. doi: 10.1074/jbc.273.22.13430. J Biol Chem. 1998. PMID: 9593675
-
The role of the trans-Golgi network in varicella zoster virus biology.Cell Mol Life Sci. 2004 Dec;61(24):3047-56. doi: 10.1007/s00018-004-4269-7. Cell Mol Life Sci. 2004. PMID: 15583866 Review.
-
Varicella-zoster virus glycoprotein M.Curr Top Microbiol Immunol. 2010;342:147-54. doi: 10.1007/82_2010_30. Curr Top Microbiol Immunol. 2010. PMID: 20373090 Review.
Cited by
-
Differential requirement for cell fusion and virion formation in the pathogenesis of varicella-zoster virus infection in skin and T cells.J Virol. 2004 Dec;78(23):13293-305. doi: 10.1128/JVI.78.23.13293-13305.2004. J Virol. 2004. PMID: 15542680 Free PMC article.
-
Impairment of nuclear pores in bovine herpesvirus 1-infected MDBK cells.J Virol. 2005 Jan;79(2):1071-83. doi: 10.1128/JVI.79.2.1071-1083.2005. J Virol. 2005. PMID: 15613336 Free PMC article.
-
Evaluation of the Immunological Efficacy of an LNP-mRNA Vaccine Prepared from Varicella Zoster Virus Glycoprotein gE with a Double-Mutated Carboxyl Terminus in Different Untranslated Regions in Mice.Vaccines (Basel). 2023 Sep 11;11(9):1475. doi: 10.3390/vaccines11091475. Vaccines (Basel). 2023. PMID: 37766151 Free PMC article.
-
Mutational analysis of open reading frames 62 and 71, encoding the varicella-zoster virus immediate-early transactivating protein, IE62, and effects on replication in vitro and in skin xenografts in the SCID-hu mouse in vivo.J Virol. 2003 May;77(10):5607-20. doi: 10.1128/jvi.77.10.5607-5620.2003. J Virol. 2003. PMID: 12719553 Free PMC article.
-
Deletion in open reading frame 49 of varicella-zoster virus reduces virus growth in human malignant melanoma cells but not in human embryonic fibroblasts.J Virol. 2007 Nov;81(22):12654-65. doi: 10.1128/JVI.01183-07. Epub 2007 Sep 12. J Virol. 2007. PMID: 17855513 Free PMC article.
References
-
- Alconada A, Bauer U, Baudoux L, Piette J, Hoflack B. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. J Biol Chem. 1998;273:13430–13436. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

