Role of NF-kappaB and myc proteins in apoptosis induced by hepatitis B virus HBx protein
- PMID: 11119591
- PMCID: PMC113915
- DOI: 10.1128/JVI.75.1.215-225.2001
Role of NF-kappaB and myc proteins in apoptosis induced by hepatitis B virus HBx protein
Abstract
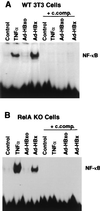
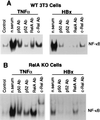
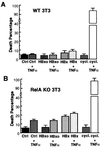
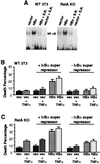
Chronic infection with hepatitis B virus (HBV) promotes a high level of liver disease and cancer in humans. The HBV HBx gene encodes a small regulatory protein that is essential for viral replication and is suspected to play a role in viral pathogenesis. HBx stimulates cytoplasmic signal transduction pathways, moderately stimulates a number of transcription factors, including several nuclear factors, and in certain settings sensitizes cells to apoptosis by proapoptotic stimuli, including tumor necrosis factor alpha (TNF-alpha) and etopocide. Paradoxically, HBx activates members of the NF-kappaB transcription factor family, some of which are antiapoptotic in function. HBx induces expression of Myc protein family members in certain settings, and Myc can sensitize cells to killing by TNF-alpha. We therefore examined the roles of NF-kappaB, c-Myc, and TNF-alpha in apoptotic killing of cells by HBx. RelA/NF-kappaB is shown to be induced by HBx and to suppress HBx-mediated apoptosis. HBx also induces c-Rel/NF-kappaB, which can promote apoptotic cell death in some contexts or block it in others. Induction of c-Rel by HBx was found to inhibit its ability to directly mediate apoptotic killing of cells. Thus, HBx induction of NF-kappaB family members masks its ability to directly mediate apoptosis, whereas ablation of NF-kappaB reveals it. Investigation of the role of Myc protein demonstrates that overexpression of Myc is essential for acute sensitization of cells to killing by HBx plus TNF-alpha. This study therefore defines a specific set of parameters which must be met for HBx to possibly contribute to HBV pathogenesis.
Figures

Similar articles
-
Synergistic and opposing regulation of the stress-responsive gene IEX-1 by p53, c-Myc, and multiple NF-kappaB/rel complexes.Oncogene. 2002 Oct 3;21(44):6819-28. doi: 10.1038/sj.onc.1205854. Oncogene. 2002. PMID: 12360408
-
Hepatitis B virus HBx protein activates transcription factor NF-kappaB by acting on multiple cytoplasmic inhibitors of rel-related proteins.J Virol. 1996 Jul;70(7):4558-66. doi: 10.1128/JVI.70.7.4558-4566.1996. J Virol. 1996. PMID: 8676482 Free PMC article.
-
Additive activation of hepatic NF-kappaB by ethanol and hepatitis B protein X (HBX) or HCV core protein: involvement of TNF-alpha receptor 1-independent and -dependent mechanisms.FASEB J. 2001 Nov;15(13):2551-3. doi: 10.1096/fj.01-0217. Epub 2001 Sep 17. FASEB J. 2001. PMID: 11641261
-
Hepatitis B virus X protein accelerates the development of hepatoma.Cancer Biol Med. 2014 Sep;11(3):182-90. doi: 10.7497/j.issn.2095-3941.2014.03.004. Cancer Biol Med. 2014. PMID: 25364579 Free PMC article. Review.
-
To die or not to die: the function of the transcription factor NF-kappaB in embryos exposed to stress.Am J Reprod Immunol. 2004 Feb;51(2):138-43. doi: 10.1046/j.8755-8920.2003.00134.x. Am J Reprod Immunol. 2004. PMID: 14748840 Review.
Cited by
-
Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response.J Virol. 2003 Dec;77(23):12852-64. doi: 10.1128/jvi.77.23.12852-12864.2003. J Virol. 2003. PMID: 14610206 Free PMC article.
-
The effects of HBx gene on the expression of DNA repair enzymes hOGG1 and hMYHalpha mRNA in HepG2 cells.J Huazhong Univ Sci Technolog Med Sci. 2009 Apr;29(2):187-92. doi: 10.1007/s11596-009-0210-5. Epub 2009 Apr 28. J Huazhong Univ Sci Technolog Med Sci. 2009. PMID: 19399402
-
Hepatitis B virus X protein regulates hepatic glucose homeostasis via activation of inducible nitric oxide synthase.J Biol Chem. 2011 Aug 26;286(34):29872-81. doi: 10.1074/jbc.M111.259978. Epub 2011 Jun 20. J Biol Chem. 2011. PMID: 21690090 Free PMC article.
-
Enhancement of gene transactivation activity of androgen receptor by hepatitis B virus X protein.Virology. 2007 Jul 5;363(2):454-61. doi: 10.1016/j.virol.2007.01.040. Epub 2007 Feb 28. Virology. 2007. PMID: 17335866 Free PMC article.
-
High level expression of apoptosis inhibitor in hepatoma cell line expressing Hepatitis B virus.Int J Med Sci. 2005;2(1):30-35. doi: 10.7150/ijms.2.30. Epub 2005 Jan 5. Int J Med Sci. 2005. PMID: 15968337 Free PMC article.
References
-
- Abbadie C, Kabrun N, Bouali F, Smardova J, Stehelin D, Vandenbunder B, Enrietto P J. High levels of c-rel expression are associated with programmed cell death in the developing avian embryo and in bone marrow cells in vitro. Cell. 1993;75:899–912. - PubMed
-
- Avantaggiati M L, Balsano C, Natoli G, DeMarzio E, Will H, Elfassi E, Levrero M. The hepatitis B virus X protein transactivation of c-fos and c-myc proto-oncogenes is mediated by multiple transcription factors. Arch Virol. 1992;4:57–61. - PubMed
-
- Baeuerle P A, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

