Origin and evolution of the mitochondrial proteome
- PMID: 11104819
- PMCID: PMC99014
- DOI: 10.1128/MMBR.64.4.786-820.2000
Origin and evolution of the mitochondrial proteome
Abstract
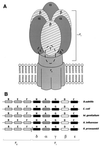
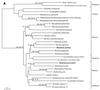
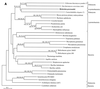
The endosymbiotic theory for the origin of mitochondria requires substantial modification. The three identifiable ancestral sources to the proteome of mitochondria are proteins descended from the ancestral alpha-proteobacteria symbiont, proteins with no homology to bacterial orthologs, and diverse proteins with bacterial affinities not derived from alpha-proteobacteria. Random mutations in the form of deletions large and small seem to have eliminated nonessential genes from the endosymbiont-mitochondrial genome lineages. This process, together with the transfer of genes from the endosymbiont-mitochondrial genome to nuclei, has led to a marked reduction in the size of mitochondrial genomes. All proteins of bacterial descent that are encoded by nuclear genes were probably transferred by the same mechanism, involving the disintegration of mitochondria or bacteria by the intracellular membranous vacuoles of cells to release nucleic acid fragments that transform the nuclear genome. This ongoing process has intermittently introduced bacterial genes to nuclear genomes. The genomes of the last common ancestor of all organisms, in particular of mitochondria, encoded cytochrome oxidase homologues. There are no phylogenetic indications either in the mitochondrial proteome or in the nuclear genomes that the initial or subsequent function of the ancestor to the mitochondria was anaerobic. In contrast, there are indications that relatively advanced eukaryotes adapted to anaerobiosis by dismantling their mitochondria and refitting them as hydrogenosomes. Accordingly, a continuous history of aerobic respiration seems to have been the fate of most mitochondrial lineages. The initial phases of this history may have involved aerobic respiration by the symbiont functioning as a scavenger of toxic oxygen. The transition to mitochondria capable of active ATP export to the host cell seems to have required recruitment of eukaryotic ATP transport proteins from the nucleus. The identity of the ancestral host of the alpha-proteobacterial endosymbiont is unclear, but there is no indication that it was an autotroph. There are no indications of a specific alpha-proteobacterial origin to genes for glycolysis. In the absence of data to the contrary, it is assumed that the ancestral host cell was a heterotroph.
Figures













Similar articles
-
MitoCOGs: clusters of orthologous genes from mitochondria and implications for the evolution of eukaryotes.BMC Evol Biol. 2014 Nov 25;14:237. doi: 10.1186/s12862-014-0237-5. BMC Evol Biol. 2014. PMID: 25421434 Free PMC article.
-
The dual origin of the yeast mitochondrial proteome.Yeast. 2000 Sep 30;17(3):170-87. doi: 10.1002/1097-0061(20000930)17:3<170::AID-YEA25>3.0.CO;2-V. Yeast. 2000. PMID: 11025528 Free PMC article.
-
Mitochondrial evolution.Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a011403. doi: 10.1101/cshperspect.a011403. Cold Spring Harb Perspect Biol. 2012. PMID: 22952398 Free PMC article. Review.
-
Mosaic nature of the mitochondrial proteome: Implications for the origin and evolution of mitochondria.Proc Natl Acad Sci U S A. 2015 Aug 18;112(33):10133-8. doi: 10.1073/pnas.1421379112. Epub 2015 Apr 6. Proc Natl Acad Sci U S A. 2015. PMID: 25848019 Free PMC article.
-
The Origin and Diversification of Mitochondria.Curr Biol. 2017 Nov 6;27(21):R1177-R1192. doi: 10.1016/j.cub.2017.09.015. Curr Biol. 2017. PMID: 29112874 Review.
Cited by
-
Borrowing nuclear DNA helicases to protect mitochondrial DNA.Int J Mol Sci. 2015 May 13;16(5):10870-87. doi: 10.3390/ijms160510870. Int J Mol Sci. 2015. PMID: 25984607 Free PMC article. Review.
-
Covariation of mitochondrial genome size with gene lengths: evidence for gene length reduction during mitochondrial evolution.J Mol Evol. 2004 Jul;59(1):90-6. doi: 10.1007/s00239-004-2607-x. J Mol Evol. 2004. PMID: 15383911
-
Structure of the yeast mitochondrial large ribosomal subunit.Science. 2014 Mar 28;343(6178):1485-1489. doi: 10.1126/science.1249410. Science. 2014. PMID: 24675956 Free PMC article.
-
Mitochondria and Aging-The Role of Exercise as a Countermeasure.Biology (Basel). 2019 May 11;8(2):40. doi: 10.3390/biology8020040. Biology (Basel). 2019. PMID: 31083586 Free PMC article. Review.
-
Structural Patching Fosters Divergence of Mitochondrial Ribosomes.Mol Biol Evol. 2019 Feb 1;36(2):207-219. doi: 10.1093/molbev/msy221. Mol Biol Evol. 2019. PMID: 30517740 Free PMC article.
References
-
- Akhmonova A, Voncken F G J, Harhangi H, Hosea K M, Vogels G D, Hackstein J H P. Cytosolic enzymes with a mitochondrial ancestry from the anaerobic chytrid Piromyces sp. E2. Mol Microbiol. 1998;30:1017–1027. - PubMed
-
- Akhmanova A, Voncken F G J, Hosea K M, Harhangi H, Keltjens J T, op den Camp H, Vogels G D, Hackstein J H P. A hydrogenosome with pyruvate formate-lyase: anaerobic chytrid fungi use an alternative route for pyruvate catabolism. Mol Microbiol. 1999;32:103–114. - PubMed
-
- Akhmanova A, Voncken F, van Alen T, van Hoek A, Boxma B, Vogels G, Veenhuis M, Hackstein J H P. A hydrogenosome with a genome. Nature (London) 1998;396:527–528. - PubMed
-
- Allen J. Control of gene expression by redox potential and the requirement for chloroplast and mitochondria genomes. J Theor Biol. 1993;165:609–631. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

