Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells
- PMID: 11101534
- PMCID: PMC305848
- DOI: 10.1093/emboj/19.23.6622
Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells
Abstract
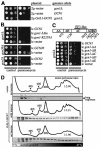
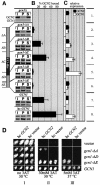
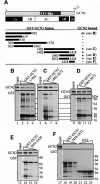
GCN2 stimulates GCN4 translation in amino acid-starved cells by phosphorylating the alpha-subunit of translation initiation factor 2. GCN2 function in vivo requires the GCN1/GCN20 complex, which binds to the N-terminal domain of GCN2. A C-terminal segment of GCN1 (residues 2052-2428) was found to be necessary and sufficient for binding GCN2 in vivo and in vitro. Overexpression of this fragment in wild-type cells impaired association of GCN2 with native GCN1 and had a dominant Gcn(-) phenotype, dependent on Arg2259 in the GCN1 fragment. Substitution of Arg2259 with Ala in full-length GCN1 abolished complex formation with native GCN2 and destroyed GCN1 regulatory function. Consistently, the Gcn(-) phenotype of gcn1-R2259A, but not that of gcn1Delta, was suppressed by overexpressing GCN2. These findings prove that GCN2 binding to the C-terminal domain of GCN1, dependent on Arg2259, is required for high level GCN2 function in vivo. GCN1 expression conferred sensitivity to paromomycin in a manner dependent on its ribosome binding domain, supporting the idea that GCN1 binds near the ribosomal acceptor site to promote GCN2 activation by uncharged tRNA.
Figures




Similar articles
-
Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2alpha kinase GCN2 is required for GCN2 activation.EMBO J. 2000 Apr 17;19(8):1887-99. doi: 10.1093/emboj/19.8.1887. EMBO J. 2000. PMID: 10775272 Free PMC article.
-
Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIF2alpha kinase GCN2.Mol Cell Biol. 1997 Aug;17(8):4474-89. doi: 10.1128/MCB.17.8.4474. Mol Cell Biol. 1997. PMID: 9234705 Free PMC article.
-
YIH1 is an actin-binding protein that inhibits protein kinase GCN2 and impairs general amino acid control when overexpressed.J Biol Chem. 2004 Jul 16;279(29):29952-62. doi: 10.1074/jbc.M404009200. Epub 2004 May 4. J Biol Chem. 2004. PMID: 15126500
-
Macromolecular mimicry in translation initiation: a model for the initiation factor IF2 on the ribosome.IUBMB Life. 2000 Dec;50(6):347-54. doi: 10.1080/713803743. IUBMB Life. 2000. PMID: 11327306 Review.
-
Emerging Role of GCN1 in Disease and Homeostasis.Int J Mol Sci. 2024 Mar 5;25(5):2998. doi: 10.3390/ijms25052998. Int J Mol Sci. 2024. PMID: 38474243 Free PMC article. Review.
Cited by
-
G-protein control of the ribosome-associated stress response protein SpoT.J Bacteriol. 2007 Sep;189(17):6140-7. doi: 10.1128/JB.00315-07. Epub 2007 Jul 6. J Bacteriol. 2007. PMID: 17616600 Free PMC article.
-
Arginase II restricts host defense to Helicobacter pylori by attenuating inducible nitric oxide synthase translation in macrophages.J Immunol. 2010 Mar 1;184(5):2572-82. doi: 10.4049/jimmunol.0902436. Epub 2010 Jan 22. J Immunol. 2010. PMID: 20097867 Free PMC article.
-
Circadian clock control of eIF2α phosphorylation is necessary for rhythmic translation initiation.Proc Natl Acad Sci U S A. 2020 May 19;117(20):10935-10945. doi: 10.1073/pnas.1918459117. Epub 2020 Apr 30. Proc Natl Acad Sci U S A. 2020. PMID: 32355000 Free PMC article.
-
Mechanism and Regulation of Protein Synthesis in Saccharomyces cerevisiae.Genetics. 2016 May;203(1):65-107. doi: 10.1534/genetics.115.186221. Genetics. 2016. PMID: 27183566 Free PMC article. Review.
-
Interaction between the tRNA-binding and C-terminal domains of Yeast Gcn2 regulates kinase activity in vivo.PLoS Genet. 2015 Feb 19;11(2):e1004991. doi: 10.1371/journal.pgen.1004991. eCollection 2015 Feb. PLoS Genet. 2015. PMID: 25695491 Free PMC article.
References
-
- Berlanga J.J., Santoyo,J. and De Haro,C. (1999) Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2α kinase. Eur. J. Biochem., 265, 754–762. - PubMed
-
- Botstein D., Falco,S.C., Stewart,S.E., Brennan,M., Scherer,S., Stinchcomb,D.T., Struhl,K. and Davis,R.W. (1979) Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene, 8, 17–24. - PubMed
-
- Broach J.R., Strathern,J.N. and Hicks,J.B. (1979) Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene, 8, 121–133. - PubMed
-
- Cashel M. and Rudd,K.E. (1987) The stringent response. In Neidhardt,F.C., Ingraham,J.L., Magasanik,B., Low,K.B., Schaechter,M. and Umbarger,H.E. (eds), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. American Society for Microbiology, Washington, DC, pp. 1410–1438.
-
- Cesareni G. and Murray,J.A.H. (1987) Plasmid vectors carrying the replication origin of filamentous single-stranded phages. In Setlow,J.K. and Hollaender,A. (eds), Genetic Engineering: Principles and Methods. Plenum Press, New York, NY, Vol. 9, pp. 135–154.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

