Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain
- PMID: 11095732
- PMCID: PMC17670
- DOI: 10.1073/pnas.250471697
Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain
Abstract
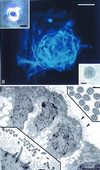
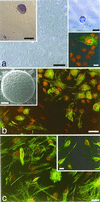
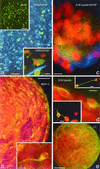
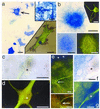
The mammalian brain contains a population of neural stem cells (NSC) that can both self-renew and generate progeny along the three lineage pathways of the central nervous system (CNS), but the in vivo identification and localization of NSC in the postnatal CNS has proved elusive. Recently, separate studies have implicated ciliated ependymal (CE) cells, and special subependymal zone (SEZ) astrocytes as candidates for NSC in the adult brain. In the present study, we have examined the potential of these two NSC candidates to form multipotent spherical clones-neurospheres-in vitro. We conclude that CE cells are unipotent and give rise only to cells within the glia cell lineage, although they are capable of forming spherical clones when cultured in isolation. In contrast, astrocyte monolayers from the cerebral cortex, cerebellum, spinal cord, and SEZ can form neurospheres that give rise both to neurons and glia. However, the ability to form neurospheres is restricted to astrocyte monolayers derived during the first 2 postnatal wk, except for SEZ astrocytes, which retain this capacity in the mature forebrain. We conclude that environmental factors, simulated by certain in vitro conditions, transiently confer NSC-like attributes on astrocytes during a critical period in CNS development.
Figures

Similar articles
-
S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage.Glia. 2007 Jan 15;55(2):165-77. doi: 10.1002/glia.20445. Glia. 2007. PMID: 17078026 Free PMC article.
-
Subventricular zone astrocytes are neural stem cells in the adult mammalian brain.Cell. 1999 Jun 11;97(6):703-16. doi: 10.1016/s0092-8674(00)80783-7. Cell. 1999. PMID: 10380923
-
A new monoclonal antibody, A3B10, specific for astrocyte-lineage cells recognizes calmodulin-regulated spectrin-associated protein 1 (Camsap1).J Neurosci Res. 2009 Feb;87(2):503-13. doi: 10.1002/jnr.21853. J Neurosci Res. 2009. PMID: 18756519
-
Proliferation and differentiation of adult endogenous neural stem cells in response to neurodegenerative process within the striatum.Neurodegener Dis. 2006;3(1-2):12-8. doi: 10.1159/000092087. Neurodegener Dis. 2006. PMID: 16909031 Review.
-
Cells in the astroglial lineage are neural stem cells.Cell Tissue Res. 2008 Jan;331(1):179-91. doi: 10.1007/s00441-007-0461-z. Epub 2007 Sep 5. Cell Tissue Res. 2008. PMID: 17786483 Review.
Cited by
-
Lin41/Trim71 is essential for mouse development and specifically expressed in postnatal ependymal cells of the brain.Front Cell Dev Biol. 2015 Apr 2;3:20. doi: 10.3389/fcell.2015.00020. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 25883935 Free PMC article.
-
Activated EGFR signaling increases proliferation, survival, and migration and blocks neuronal differentiation in post-natal neural stem cells.J Neurooncol. 2010 May;97(3):323-37. doi: 10.1007/s11060-009-0035-x. Epub 2009 Oct 24. J Neurooncol. 2010. PMID: 19855928
-
Phenotypic and functional characterization of adult brain neuropoiesis.Proc Natl Acad Sci U S A. 2005 Jun 28;102(26):9353-8. doi: 10.1073/pnas.0503965102. Epub 2005 Jun 16. Proc Natl Acad Sci U S A. 2005. PMID: 15961540 Free PMC article.
-
FoxO3 Modulates Circadian Rhythms in Neural Stem Cells.Int J Mol Sci. 2023 Sep 4;24(17):13662. doi: 10.3390/ijms241713662. Int J Mol Sci. 2023. PMID: 37686468 Free PMC article.
-
New Neurons in the Post-ischemic and Injured Brain: Migrating or Resident?Front Neurosci. 2019 Jun 18;13:588. doi: 10.3389/fnins.2019.00588. eCollection 2019. Front Neurosci. 2019. PMID: 31275097 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

