Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo
- PMID: 11087820
- PMCID: PMC17626
- DOI: 10.1073/pnas.240309797
Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo
Abstract
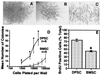
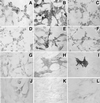
Dentinal repair in the postnatal organism occurs through the activity of specialized cells, odontoblasts, that are thought to be maintained by an as yet undefined precursor population associated with pulp tissue. In this study, we isolated a clonogenic, rapidly proliferative population of cells from adult human dental pulp. These DPSCs were then compared with human bone marrow stromal cells (BMSCs), known precursors of osteoblasts. Although they share a similar immunophenotype in vitro, functional studies showed that DPSCs produced only sporadic, but densely calcified nodules, and did not form adipocytes, whereas BMSCs routinely calcified throughout the adherent cell layer with clusters of lipid-laden adipocytes. When DPSCs were transplanted into immunocompromised mice, they generated a dentin-like structure lined with human odontoblast-like cells that surrounded a pulp-like interstitial tissue. In contrast, BMSCs formed lamellar bone containing osteocytes and surface-lining osteoblasts, surrounding a fibrous vascular tissue with active hematopoiesis and adipocytes. This study isolates postnatal human DPSCs that have the ability to form a dentin/pulp-like complex.
Figures




Similar articles
-
A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB).J Bone Miner Res. 2005 Aug;20(8):1394-402. doi: 10.1359/JBMR.050325. Epub 2005 Mar 28. J Bone Miner Res. 2005. PMID: 16007337
-
Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium.Tissue Eng. 2006 Nov;12(11):3097-105. doi: 10.1089/ten.2006.12.3097. Tissue Eng. 2006. PMID: 17518625
-
A method to isolate and culture expand human dental pulp stem cells.Methods Mol Biol. 2011;698:107-21. doi: 10.1007/978-1-60761-999-4_9. Methods Mol Biol. 2011. PMID: 21431514
-
Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex.Congenit Anom (Kyoto). 2016 Jul;56(4):144-53. doi: 10.1111/cga.12169. Congenit Anom (Kyoto). 2016. PMID: 27131345 Review.
-
Dental pulp stem cells: function, isolation and applications in regenerative medicine.J Tissue Eng Regen Med. 2015 Nov;9(11):1205-16. doi: 10.1002/term.1899. Epub 2014 May 21. J Tissue Eng Regen Med. 2015. PMID: 24850632 Review.
Cited by
-
Concise review: personalized human bone grafts for reconstructing head and face.Stem Cells Transl Med. 2012 Jan;1(1):64-9. doi: 10.5966/sctm.2011-0020. Epub 2011 Dec 7. Stem Cells Transl Med. 2012. PMID: 23197642 Free PMC article. Review.
-
Human adult dental pulp stem cells enhance poststroke functional recovery through non-neural replacement mechanisms.Stem Cells Transl Med. 2012 Mar;1(3):177-87. doi: 10.5966/sctm.2011-0039. Epub 2012 Feb 22. Stem Cells Transl Med. 2012. PMID: 23197777 Free PMC article.
-
Therapeutic potential of dental pulp stem cells and their derivatives: Insights from basic research toward clinical applications.World J Stem Cells. 2022 Jul 26;14(7):435-452. doi: 10.4252/wjsc.v14.i7.435. World J Stem Cells. 2022. PMID: 36157522 Free PMC article. Review.
-
Characterization of α-smooth muscle actin positive cells during multilineage differentiation of dental pulp stem cells.Cell Prolif. 2012 Jun;45(3):259-65. doi: 10.1111/j.1365-2184.2012.00818.x. Epub 2012 Apr 8. Cell Prolif. 2012. PMID: 22487297 Free PMC article.
-
Comparative characterization and analysis of telomere length in stem cells derived from deciduous and permanent teeth.Dent Res J (Isfahan). 2022 Aug 16;19:64. eCollection 2022. Dent Res J (Isfahan). 2022. PMID: 36159052 Free PMC article.
References
-
- Smith A J, Cassidy N, Perry H, Begue-Kirn C, Ruch J V, Lesot H. Int J Dev Biol. 1995;39:273–280. - PubMed
-
- Kitamura C, Kimura K, Nakayama T, Terashita M. J Dent Res. 1999;78:673–680. - PubMed
-
- Ruch J V. Biochem Cell Biol. 1998;76:923–938. - PubMed
-
- Couble M L, Farges J C, Bleicher F, Perrat-Mabillon B, Boudeulle M, Magloire H. Calcif Tissue Int. 2000;66:129–138. - PubMed
-
- Cheng S L, Yang J W, Rifas L, Zhang S F, Avioli L V. Endocrinology. 1994;134:277–286. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

