Dissection of autophagosome biogenesis into distinct nucleation and expansion steps
- PMID: 11086004
- PMCID: PMC2174351
- DOI: 10.1083/jcb.151.5.1025
Dissection of autophagosome biogenesis into distinct nucleation and expansion steps
Abstract
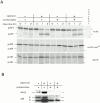
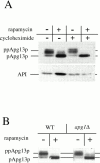
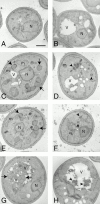
Rapamycin, an antifungal macrolide antibiotic, mimics starvation conditions in Saccharomyces cerevisiae through activation of a general G(0) program that includes widespread effects on translation and transcription. Macroautophagy, a catabolic membrane trafficking phenomenon, is a prominent part of this response. Two views of the induction of autophagy may be considered. In one, up-regulation of proteins involved in autophagy causes its induction, implying that autophagy is the result of a signal transduction mechanism leading from Tor to the transcriptional and translational machinery. An alternative hypothesis postulates the existence of a dedicated signal transduction mechanism that induces autophagy directly. We tested these possibilities by assaying the effects of cycloheximide and specific mutations on the induction of autophagy. We find that induction of autophagy takes place in the absence of de novo protein synthesis, including that of specific autophagy-related proteins that are up-regulated in response to rapamycin. We also find that dephosphorylation of Apg13p, a signal transduction event that correlates with the onset of autophagy, is also independent of new protein synthesis. Finally, our data indicate that autophagosomes that form in the absence of protein synthesis are significantly smaller than normal, indicating a role for de novo protein synthesis in the regulation of autophagosome expansion. Our results define the existence of a signal transduction-dependent nucleation step and a separate autophagosome expansion step that together coordinate autophagosome biogenesis.
Figures

Similar articles
-
Tor-mediated induction of autophagy via an Apg1 protein kinase complex.J Cell Biol. 2000 Sep 18;150(6):1507-13. doi: 10.1083/jcb.150.6.1507. J Cell Biol. 2000. PMID: 10995454 Free PMC article.
-
Apg13p and Vac8p are part of a complex of phosphoproteins that are required for cytoplasm to vacuole targeting.J Biol Chem. 2000 Aug 18;275(33):25840-9. doi: 10.1074/jbc.M002813200. J Biol Chem. 2000. PMID: 10837477
-
Autophagy in yeast: a TOR-mediated response to nutrient starvation.Curr Top Microbiol Immunol. 2004;279:73-84. doi: 10.1007/978-3-642-18930-2_5. Curr Top Microbiol Immunol. 2004. PMID: 14560952 Review.
-
Tor pathway control of the nitrogen-responsive DAL5 gene bifurcates at the level of Gln3 and Gat1 regulation in Saccharomyces cerevisiae.J Biol Chem. 2008 Apr 4;283(14):8919-29. doi: 10.1074/jbc.M708811200. Epub 2008 Feb 1. J Biol Chem. 2008. PMID: 18245087 Free PMC article.
-
Elucidating TOR signaling and rapamycin action: lessons from Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2002 Dec;66(4):579-91, table of contents. doi: 10.1128/MMBR.66.4.579-591.2002. Microbiol Mol Biol Rev. 2002. PMID: 12456783 Free PMC article. Review.
Cited by
-
Atg29 phosphorylation regulates coordination of the Atg17-Atg31-Atg29 complex with the Atg11 scaffold during autophagy initiation.Proc Natl Acad Sci U S A. 2013 Jul 30;110(31):E2875-84. doi: 10.1073/pnas.1300064110. Epub 2013 Jul 15. Proc Natl Acad Sci U S A. 2013. PMID: 23858448 Free PMC article.
-
Membrane recruitment of Aut7p in the autophagy and cytoplasm to vacuole targeting pathways requires Aut1p, Aut2p, and the autophagy conjugation complex.J Cell Biol. 2001 Jan 8;152(1):51-64. doi: 10.1083/jcb.152.1.51. J Cell Biol. 2001. PMID: 11149920 Free PMC article.
-
Atg21 is a phosphoinositide binding protein required for efficient lipidation and localization of Atg8 during uptake of aminopeptidase I by selective autophagy.Mol Biol Cell. 2004 Aug;15(8):3553-66. doi: 10.1091/mbc.e04-02-0147. Epub 2004 May 21. Mol Biol Cell. 2004. PMID: 15155809 Free PMC article.
-
Vacuolar localization of oligomeric alpha-mannosidase requires the cytoplasm to vacuole targeting and autophagy pathway components in Saccharomyces cerevisiae.J Biol Chem. 2001 Jun 8;276(23):20491-8. doi: 10.1074/jbc.M101150200. Epub 2001 Mar 22. J Biol Chem. 2001. PMID: 11264288 Free PMC article.
-
A novel ATG4B antagonist inhibits autophagy and has a negative impact on osteosarcoma tumors.Autophagy. 2014;10(11):2021-35. doi: 10.4161/auto.32229. Epub 2014 Oct 30. Autophagy. 2014. PMID: 25483883 Free PMC article.
References
-
- Abeliovich H., Grote E., Novick P., Ferro-Novick S. Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J. Biol. Chem. 1998;273:11719–11727. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

