Virus-encoded suppressor of posttranscriptional gene silencing targets a maintenance step in the silencing pathway
- PMID: 11078509
- PMCID: PMC27236
- DOI: 10.1073/pnas.230334397
Virus-encoded suppressor of posttranscriptional gene silencing targets a maintenance step in the silencing pathway
Abstract
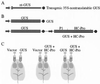
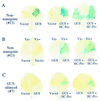
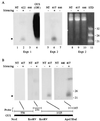
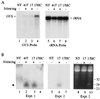
Certain plant viruses encode suppressors of posttranscriptional gene silencing (PTGS), an adaptive antiviral defense response that limits virus replication and spread. The tobacco etch potyvirus protein, helper component-proteinase (HC-Pro), suppresses PTGS of silenced transgenes. The effect of HC-Pro on different steps of the silencing pathway was analyzed by using both transient Agrobacterium tumefaciens-based delivery and transgenic systems. HC-Pro inactivated PTGS in plants containing a preexisting silenced beta-glucuronidase (GUS) transgene. PTGS in this system was associated with both small RNA molecules (21-26 nt) corresponding to the 3' proximal region of the transcribed GUS sequence and cytosine methylation of specific sites near the 3' end of the GUS transgene. Introduction of HC-Pro into these plants resulted in loss of PTGS, loss of small RNAs, and partial loss of methylation. These results suggest that HC-Pro targets a PTGS maintenance (as opposed to an initiation or signaling) component at a point that affects accumulation of small RNAs and methylation of genomic DNA.
Figures

Similar articles
-
HC-Pro suppression of transgene silencing eliminates the small RNAs but not transgene methylation or the mobile signal.Plant Cell. 2001 Mar;13(3):571-83. doi: 10.1105/tpc.13.3.571. Plant Cell. 2001. PMID: 11251097 Free PMC article.
-
Long-distance movement and replication maintenance functions correlate with silencing suppression activity of potyviral HC-Pro.Virology. 2001 Jun 20;285(1):71-81. doi: 10.1006/viro.2001.0901. Virology. 2001. PMID: 11414807
-
A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants.Science. 2000 Oct 6;290(5489):142-4. doi: 10.1126/science.290.5489.142. Science. 2000. PMID: 11021800
-
A viral protein suppresses siRNA-directed interference in tobacco mosaic virus infection.Acta Biochim Biophys Sin (Shanghai). 2005 Apr;37(4):248-53. doi: 10.1111/j.1745-7270.2005.00036.x. Acta Biochim Biophys Sin (Shanghai). 2005. PMID: 15806291
-
Potyviral Helper-Component Protease: Multifaced Functions and Interactions with Host Proteins.Plants (Basel). 2024 Apr 29;13(9):1236. doi: 10.3390/plants13091236. Plants (Basel). 2024. PMID: 38732454 Free PMC article. Review.
Cited by
-
P2 of Rice grassy stunt virus (RGSV) and p6 and p9 of Rice ragged stunt virus (RRSV) isolates from Vietnam exert suppressor activity on the RNA silencing pathway.Virus Genes. 2015 Oct;51(2):267-75. doi: 10.1007/s11262-015-1229-2. Epub 2015 Jul 28. Virus Genes. 2015. PMID: 26215087
-
Identification of an RNA silencing suppressor from a plant double-stranded RNA virus.J Virol. 2005 Oct;79(20):13018-27. doi: 10.1128/JVI.79.20.13018-13027.2005. J Virol. 2005. PMID: 16189004 Free PMC article.
-
Control of nuclear and nucleolar localization of nuclear inclusion protein a of picorna-like Potato virus A in Nicotiana species.Plant Cell. 2009 Aug;21(8):2485-502. doi: 10.1105/tpc.108.064147. Epub 2009 Aug 21. Plant Cell. 2009. PMID: 19700632 Free PMC article.
-
Replication-independent long-distance trafficking by viral RNAs in Nicotiana benthamiana.Plant Cell. 2007 Apr;19(4):1179-91. doi: 10.1105/tpc.107.050088. Epub 2007 Apr 6. Plant Cell. 2007. PMID: 17416731 Free PMC article.
-
Negative-strand tospoviruses and tenuiviruses carry a gene for a suppressor of gene silencing at analogous genomic positions.J Virol. 2003 Jan;77(2):1329-36. doi: 10.1128/jvi.77.2.1329-1336.2003. J Virol. 2003. PMID: 12502849 Free PMC article.
References
-
- Sijen T, Kooter J M. BioEssays. 2000;22:520–531. - PubMed
-
- Bass B L. Cell. 2000;101:235–238. - PubMed
-
- Hamilton A J, Baulcombe D C. Science. 1999;286:950–952. - PubMed
-
- Hammond S M, Bernstein E, Beach D, Hannon G J. Nature (London) 2000;404:293–296. - PubMed
-
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe D C. Cell. 2000;101:543–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

