Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12
- PMID: 11073979
- PMCID: PMC86511
- DOI: 10.1128/MCB.20.23.8783-8792.2000
Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12
Abstract
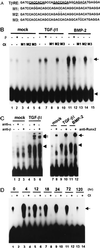
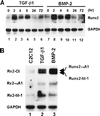
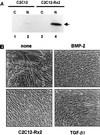
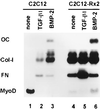
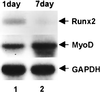
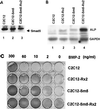
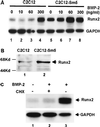
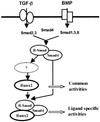
When C2C12 pluripotent mesenchymal precursor cells are treated with transforming growth factor beta1 (TGF-beta1), terminal differentiation into myotubes is blocked. Treatment with bone morphogenetic protein 2 (BMP-2) not only blocks myogenic differentiation of C2C12 cells but also induces osteoblast differentiation. The molecular mechanisms governing the ability of TGF-beta1 and BMP-2 to both induce ligand-specific responses and inhibit myogenic differentiation are not known. We identified Runx2/PEBP2alphaA/Cbfa1, a global regulator of osteogenesis, as a major TGF-beta1-responsive element binding protein induced by TGF-beta1 and BMP-2 in C2C12 cells. Consistent with the observation that Runx2 can be induced by either TGF-beta1 or BMP-2, the exogenous expression of Runx2 mediated some of the effects of TGF-beta1 and BMP-2 but not osteoblast-specific gene expression. Runx2 mimicked common effects of TGF-beta1 and BMP-2 by inducing expression of matrix gene products (for example, collagen and fibronectin), suppressing MyoD expression, and inhibiting myotube formation of C2C12 cells. For osteoblast differentiation, an additional effector, BMP-specific Smad protein, was required. Our results indicate that Runx2 is a major target gene shared by TGF-beta and BMP signaling pathways and that the coordinated action of Runx2 and BMP-activated Smads leads to the induction of osteoblast-specific gene expression in C2C12 cells.
Figures

Similar articles
-
Core-binding factor alpha 1 (Cbfa1) induces osteoblastic differentiation of C2C12 cells without interactions with Smad1 and Smad5.Bone. 2002 Aug;31(2):303-12. doi: 10.1016/s8756-3282(02)00826-8. Bone. 2002. PMID: 12151083
-
Intimate relationship between TGF-beta/BMP signaling and runt domain transcription factor, PEBP2/CBF.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S48-55. J Bone Joint Surg Am. 2001. PMID: 11263665 Review.
-
Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein.Oncogene. 2002 Oct 17;21(47):7156-63. doi: 10.1038/sj.onc.1205937. Oncogene. 2002. PMID: 12370805
-
Smad1 and smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts.Biochem Biophys Res Commun. 1997 Sep 18;238(2):574-80. doi: 10.1006/bbrc.1997.7325. Biochem Biophys Res Commun. 1997. PMID: 9299554
-
Smad-Runx interactions during chondrocyte maturation.J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 1):S15-22. J Bone Joint Surg Am. 2001. PMID: 11263661 Review.
Cited by
-
Unveiling the Role of Sik1 in Osteoblast Differentiation: Implications for Osteoarthritis.Mol Cell Biol. 2024;44(10):411-428. doi: 10.1080/10985549.2024.2385633. Epub 2024 Aug 22. Mol Cell Biol. 2024. PMID: 39169784 Free PMC article.
-
Ameloblastin upstream region contains structural elements regulating transcriptional activity in a stromal cell line derived from bone marrow.Eur J Oral Sci. 2011 Dec;119 Suppl 1(Suppl 1):286-92. doi: 10.1111/j.1600-0722.2011.00910.x. Eur J Oral Sci. 2011. PMID: 22243258 Free PMC article.
-
Inorganic phosphate-osteogenic induction medium promotes osteogenic differentiation of valvular interstitial cells via the BMP-2/Smad1/5/9 and RhoA/ROCK-1 signaling pathways.Am J Transl Res. 2020 Jul 15;12(7):3329-3345. eCollection 2020. Am J Transl Res. 2020. PMID: 32774703 Free PMC article.
-
BRITER: a BMP responsive osteoblast reporter cell line.PLoS One. 2012;7(5):e37134. doi: 10.1371/journal.pone.0037134. Epub 2012 May 14. PLoS One. 2012. PMID: 22611465 Free PMC article.
-
Organizational metrics of interchromatin speckle factor domains: integrative classifier for stem cell adhesion & lineage signaling.Integr Biol (Camb). 2015 Apr;7(4):435-46. doi: 10.1039/c4ib00281d. Integr Biol (Camb). 2015. PMID: 25765854 Free PMC article.
References
-
- Akiyama S, Katagiri T, Namiki M, Yamaji N, Yamamoto N, Miyama K, Shibuya H, Ueno N, Wozney J M, Suda T. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp Cell Res. 1997;235:362–369. - PubMed
-
- Bae S C, Yamaguchi-Iwai Y, Ogawa E, Maruyama M, Inuzuka M, Kagoshima H, Shigesada K, Satake M, Ito Y. Isolation of PEBP2 αB cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993;8:809–814. - PubMed
-
- Bae S C, Ito Y. Regulation mechanisms for the heterodimeric transcription factor, PEBP2/CBF. Histol Histopathol. 1999;14:1213–1221. - PubMed
-
- Banerjee C, McCabe L R, Choi J Y, Hiebert S W, Stein J L, Stein G S, Lian J B. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone specific complex. J Cell Biochem. 1997;66:1–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
