Catalase-peroxidases of Legionella pneumophila: cloning of the katA gene and studies of KatA function
- PMID: 11073912
- PMCID: PMC111410
- DOI: 10.1128/JB.182.23.6679-6686.2000
Catalase-peroxidases of Legionella pneumophila: cloning of the katA gene and studies of KatA function
Abstract
Legionella pneumophila, the causative organism of Legionnaires' pneumonia, contains two enzymes with catalatic and peroxidatic activity, KatA and KatB. To address the issue of redundant, overlapping, or discrete in vivo functions of highly homologous catalase-peroxidases, the gene for katA was cloned and its function was studied in L. pneumophila and Escherichia coli and compared with prior studies of katB in this laboratory. katA is induced during exponential growth and is the predominant peroxidase in stationary phase. When katA is inactivated, L. pneumophila is more sensitive to exogenous hydrogen peroxide and less virulent in the THP-1 macrophage cell line, similar to katB. Catalatic-peroxidatic activity with different peroxidatic cosubstrates is comparable for KatA and KatB, but KatA is five times more active towards dianisidine. In contrast with these examples of redundant or overlapping function, stationary-phase survival is decreased by 100- to 10,000-fold when katA is inactivated, while no change from wild type is seen for the katB null. The principal clue for understanding this discrete in vivo function was the demonstration that KatA is periplasmic and KatB is cytosolic. This stationary-phase phenotype suggests that targets sensitive to hydrogen peroxide are present outside the cytosol in stationary phase or that the peroxidatic activity of KatA is critical for stationary-phase redox reactions in the periplasm, perhaps disulfide bond formation. Since starvation-induced stationary phase is a prerequisite to acquisition of virulence by L. pneumophila, further studies on the function and regulation of katA in stationary phase may give insights on the mechanisms of infectivity of this pathogen.
Figures

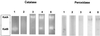
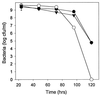
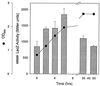
Similar articles
-
Legionella pneumophila catalase-peroxidases: cloning of the katB gene and studies of KatB function.J Bacteriol. 1998 Oct;180(20):5369-74. doi: 10.1128/JB.180.20.5369-5374.1998. J Bacteriol. 1998. PMID: 9765568 Free PMC article.
-
Legionella pneumophila catalase-peroxidases are required for proper trafficking and growth in primary macrophages.Infect Immun. 2003 Aug;71(8):4526-35. doi: 10.1128/IAI.71.8.4526-4535.2003. Infect Immun. 2003. PMID: 12874332 Free PMC article.
-
Periplasmic copper-zinc superoxide dismutase of Legionella pneumophila: role in stationary-phase survival.J Bacteriol. 1996 Mar;178(6):1578-84. doi: 10.1128/jb.178.6.1578-1584.1996. J Bacteriol. 1996. PMID: 8626284 Free PMC article.
-
Pathogenicity of Legionella pneumophila.Int J Med Microbiol. 2001 Nov;291(5):331-43. doi: 10.1078/1438-4221-00139. Int J Med Microbiol. 2001. PMID: 11727817 Review.
-
Genetic approaches to study Legionella pneumophila pathogenicity.FEMS Microbiol Rev. 1994 Jun;14(2):161-76. doi: 10.1111/j.1574-6976.1994.tb00085.x. FEMS Microbiol Rev. 1994. PMID: 8049098 Review.
Cited by
-
Virulence phenotypes of Legionella pneumophila associated with noncoding RNA lpr0035.Infect Immun. 2012 Dec;80(12):4143-53. doi: 10.1128/IAI.00598-12. Epub 2012 Sep 10. Infect Immun. 2012. PMID: 22966048 Free PMC article.
-
An ortholog of OxyR in Legionella pneumophila is expressed postexponentially and negatively regulates the alkyl hydroperoxide reductase (ahpC2D) operon.J Bacteriol. 2008 May;190(10):3444-55. doi: 10.1128/JB.00141-08. Epub 2008 Mar 21. J Bacteriol. 2008. PMID: 18359810 Free PMC article.
-
Non-classical protein secretion in bacteria.BMC Microbiol. 2005 Oct 7;5:58. doi: 10.1186/1471-2180-5-58. BMC Microbiol. 2005. PMID: 16212653 Free PMC article.
-
Environmental mimics and the Lvh type IVA secretion system contribute to virulence-related phenotypes of Legionella pneumophila.Infect Immun. 2007 Feb;75(2):723-35. doi: 10.1128/IAI.00956-06. Epub 2006 Nov 13. Infect Immun. 2007. PMID: 17101653 Free PMC article.
-
Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli.J Bacteriol. 2001 Dec;183(24):7182-9. doi: 10.1128/JB.183.24.7182-7189.2001. J Bacteriol. 2001. PMID: 11717277 Free PMC article.
References
-
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;108:121–126. - PubMed
-
- Amemura-Maekawa J, Mishima-Abe S, Kura F, Takahashi T, Watanabe H. Identification of a novel periplasmic catalase-peroxidase KatA of Legionella pneumophila. FEMS Microbiol Lett. 1999;176:339–344. - PubMed
-
- Bader M, Muse W, Ballou D P, Gassner C, Bardwell J C. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

