Determining the role of the Epstein-Barr virus Cp EBNA2-dependent enhancer during the establishment of latency by using mutant and wild-type viruses recovered from cottontop marmoset lymphoblastoid cell lines
- PMID: 11070007
- PMCID: PMC113192
- DOI: 10.1128/jvi.74.23.11115-11120.2000
Determining the role of the Epstein-Barr virus Cp EBNA2-dependent enhancer during the establishment of latency by using mutant and wild-type viruses recovered from cottontop marmoset lymphoblastoid cell lines
Abstract
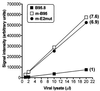
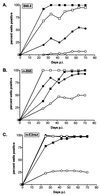
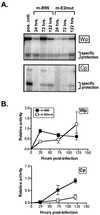
Epstein-Barr virus (EBV) nuclear antigen (EBNA) 2 (EBNA2) is involved in upregulating the expression of both EBNAs and latency-associated membrane proteins. Transcription of the six EBNA genes, which are expressed in EBV-immortalized primary B cells, arises from one of two promoters, Cp and Wp, located near the left end of the viral genome. Wp is exclusively used to drive EBNA gene transcription during the initial stages of infection in primary B cells; induction of transcription from Cp follows. We previously have mapped an EBNA2-dependent enhancer upstream of Cp (M. Woisetschlaeger et al., Proc. Natl. Acad. Sci. USA 88:3942-3946, 1991) and, more recently, have demonstrated that deletion of this enhancer results in EBV-immortalized lymphoblastoid cell lines (LCLs) that are heavily biased toward the use of Wp to drive transcription of the EBNA genes (L. Yoo et al., J. Virol. 71:9134-9142, 1997). To assess the immortalizing capacity of this mutant EBV and to monitor the early events after infection of primary B cells, B cells isolated from cottontop marmosets were used to generate LCLs immortalized with the Cp EBNA2 enhancer deletion mutant virus. As previously reported, all EBV-infected marmoset LCLs examined could be triggered to produce significant levels of virus. Infection of human B cells with wild-type or Cp EBNA2 enhancer mutant viruses recovered from marmoset B-cell lines demonstrated that (i) the Cp EBNA2 enhancer mutant virus immortalizes primary human B cells nearly as efficiently as wild-type virus and (ii) the Cp EBNA2-dependent enhancer plays an important role in the induction of Cp activity during the early stages of infection. The latter is consistent with the phenotype of LCLs immortalized with the Cp EBNA2 enhancer mutant EBV. Finally, using an established LCL in which EBNA2 function is regulated by beta-estradiol, we showed that the loss of EBNA2 function results in an approximately 4-fold decrease in the steady-state levels of Cp-initiated transcripts and a concomitant increase in the steady-state levels of Wp-initiated transcripts. Taken together, these results provide strong evidence that EBNA2 plays an important role in regulating Cp activity. These results also demonstrate that diminished induction of Cp activity does not appear to affect the ability of EBV to immortalize primary B cells in cultures. Finally, as shown here, infection of marmoset B cells with immortalization-competent mutants of EBV provides a convenient reservoir for the production of mutant viruses.
Figures

Similar articles
-
B-cell lines immortalized with an Epstein-Barr virus mutant lacking the Cp EBNA2 enhancer are biased toward utilization of the oriP-proximal EBNA gene promoter Wp1.J Virol. 1997 Dec;71(12):9134-42. doi: 10.1128/JVI.71.12.9134-9142.1997. J Virol. 1997. PMID: 9371570 Free PMC article.
-
Transcription of the Epstein-Barr virus nuclear antigen 1 (EBNA1) gene occurs before induction of the BCR2 (Cp) EBNA gene promoter during the initial stages of infection in B cells.J Virol. 1996 Jun;70(6):3561-70. doi: 10.1128/JVI.70.6.3561-3570.1996. J Virol. 1996. PMID: 8648690 Free PMC article.
-
oriP is essential for EBNA gene promoter activity in Epstein-Barr virus-immortalized lymphoblastoid cell lines.J Virol. 1996 Sep;70(9):5758-68. doi: 10.1128/JVI.70.9.5758-5768.1996. J Virol. 1996. PMID: 8709191 Free PMC article.
-
Epigenetic regulation of latent Epstein-Barr virus promoters.Biochim Biophys Acta. 2010 Mar-Apr;1799(3-4):228-35. doi: 10.1016/j.bbagrm.2009.10.005. Epub 2009 Oct 22. Biochim Biophys Acta. 2010. PMID: 19853674 Review.
-
Immortalizing genes of Epstein-Barr virus.Adv Virus Res. 1991;40:19-55. doi: 10.1016/s0065-3527(08)60276-6. Adv Virus Res. 1991. PMID: 1659776 Review.
Cited by
-
The Epstein-Barr virus BamHI C promoter is not essential for B cell immortalization in vitro, but it greatly enhances B cell growth transformation.J Virol. 2015 Mar;89(5):2483-93. doi: 10.1128/JVI.03300-14. Epub 2014 Dec 24. J Virol. 2015. PMID: 25540367 Free PMC article.
-
Dynamic chromatin boundaries delineate a latency control region of Epstein-Barr virus.J Virol. 2004 Nov;78(22):12308-19. doi: 10.1128/JVI.78.22.12308-12319.2004. J Virol. 2004. PMID: 15507618 Free PMC article.
-
Deletion of Epstein-Barr virus regulatory sequences upstream of the EBNA gene promoter Wp1 is unfavorable for B-Cell immortalization.J Virol. 2002 Nov;76(22):11763-9. doi: 10.1128/jvi.76.22.11763-11769.2002. J Virol. 2002. PMID: 12388739 Free PMC article.
-
trans-Repression of protein expression dependent on the Epstein-Barr virus promoter Wp during latency.J Virol. 2011 Nov;85(21):11435-47. doi: 10.1128/JVI.05158-11. Epub 2011 Aug 24. J Virol. 2011. PMID: 21865378 Free PMC article.
-
Epigenetic deregulation of the LMP1/LMP2 locus of Epstein-Barr virus by mutation of a single CTCF-cohesin binding site.J Virol. 2014 Feb;88(3):1703-13. doi: 10.1128/JVI.02209-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257606 Free PMC article.
References
-
- Alfieri C, Birkenbach M, Kieff E. Early events in Epstein-Barr virus infection of human B lymphocytes. Virology. 1991;181:595–608. . (Erratum, 185:946.) - PubMed
-
- Ben Sasson S A, Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981;28:131–135. - PubMed
-
- Chang R S, Lung M L. A modified procedure for the propagation of wild-type Epstein-Barr virus in cultures of marmoset blood cells. J Virol Methods. 1994;46:167–178. - PubMed
-
- Desgranges C, Lenoir G, de The G, Seigneurin J M, Hilgers J, Dubouch P. In vitro transforming activity of EBV. I. Establishment and properties of two EBV strains (M81 and M72) produced by immortalized Callithrix jacchus lymphocytes. Biomedicine. 1976;25:349–352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

