Structure of lipid bilayers
- PMID: 11063882
- PMCID: PMC2747654
- DOI: 10.1016/s0304-4157(00)00016-2
Structure of lipid bilayers
Abstract
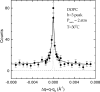
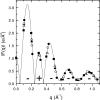
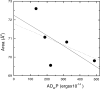
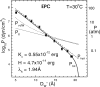
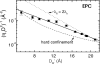
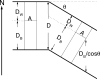
The quantitative experimental uncertainty in the structure of fully hydrated, biologically relevant, fluid (L(alpha)) phase lipid bilayers has been too large to provide a firm base for applications or for comparison with simulations. Many structural methods are reviewed including modern liquid crystallography of lipid bilayers that deals with the fully developed undulation fluctuations that occur in the L(alpha) phase. These fluctuations degrade the higher order diffraction data in a way that, if unrecognized, leads to erroneous conclusions regarding bilayer structure. Diffraction measurements at high instrumental resolution provide a measure of these fluctuations. In addition to providing better structural determination, this opens a new window on interactions between bilayers, so the experimental determination of interbilayer interaction parameters is reviewed briefly. We introduce a new structural correction based on fluctuations that has not been included in any previous studies. Updated measurements, such as for the area compressibility modulus, are used to provide adjustments to many of the literature values of structural quantities. Since the gel (L(beta)') phase is valuable as a stepping stone for obtaining fluid phase results, a brief review is given of the lower temperature phases. The uncertainty in structural results for lipid bilayers is being reduced and best current values are provided for bilayers of five lipids.
Figures

Similar articles
-
Structure and interactions of fully hydrated dioleoylphosphatidylcholine bilayers.Biophys J. 1998 Aug;75(2):917-25. doi: 10.1016/S0006-3495(98)77580-0. Biophys J. 1998. PMID: 9675192 Free PMC article.
-
Contributions of hydration and steric (entropic) pressures to the interactions between phosphatidylcholine bilayers: experiments with the subgel phase.Biochemistry. 1993 Aug 17;32(32):8374-84. doi: 10.1021/bi00083a042. Biochemistry. 1993. PMID: 8347634
-
X-ray structure determination of fully hydrated L alpha phase dipalmitoylphosphatidylcholine bilayers.Biophys J. 1996 Mar;70(3):1419-31. doi: 10.1016/S0006-3495(96)79701-1. Biophys J. 1996. PMID: 8785298 Free PMC article.
-
Lipid bilayers: thermodynamics, structure, fluctuations, and interactions.Chem Phys Lipids. 2004 Jan;127(1):3-14. doi: 10.1016/j.chemphyslip.2003.09.002. Chem Phys Lipids. 2004. PMID: 14706737 Free PMC article. Review.
-
Elastic deformation and area per lipid of membranes: atomistic view from solid-state deuterium NMR spectroscopy.Biochim Biophys Acta. 2015 Jan;1848(1 Pt B):246-59. doi: 10.1016/j.bbamem.2014.06.004. Epub 2014 Jun 16. Biochim Biophys Acta. 2015. PMID: 24946141 Free PMC article. Review.
Cited by
-
Understanding the influence of lipid bilayers and ligand molecules in determining the conformational dynamics of somatostatin receptor 2.Sci Rep. 2021 Apr 7;11(1):7677. doi: 10.1038/s41598-021-87422-5. Sci Rep. 2021. PMID: 33828200 Free PMC article.
-
Dioctadecyldimethylammonium bromide, a surfactant model for the cell membrane: Importance of microscopic dynamics.Struct Dyn. 2020 Sep 22;7(5):051301. doi: 10.1063/4.0000030. eCollection 2020 Sep. Struct Dyn. 2020. PMID: 32984433 Free PMC article. Review.
-
Structural basis of synaptic vesicle assembly promoted by α-synuclein.Nat Commun. 2016 Sep 19;7:12563. doi: 10.1038/ncomms12563. Nat Commun. 2016. PMID: 27640673 Free PMC article.
-
Lipid bilayer properties potentially contributed to the evolutionary disappearance of betaine lipids in seed plants.BMC Biol. 2023 Nov 28;21(1):275. doi: 10.1186/s12915-023-01775-z. BMC Biol. 2023. PMID: 38017456 Free PMC article.
-
Calcium enhances binding of Aβ monomer to DMPC lipid bilayer.Biophys J. 2015 Apr 7;108(7):1807-1818. doi: 10.1016/j.bpj.2015.03.001. Biophys J. 2015. PMID: 25863071 Free PMC article.
References
-
- Rand RP, Parsegian VA. Biochim. Biophys. Acta. 1989;988:351–376.
-
- Sun W-J, Suter RM, Knewtson MA, Worthington CR, Tristram-Nagle S, Zhang R, Nagle JF. Phys. Rev. E. 1994;49:4665–4676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

