Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2
- PMID: 11050165
- PMCID: PMC18808
- DOI: 10.1073/pnas.220423497
Peripheral Golgi protein GRASP65 is a target of mitotic polo-like kinase (Plk) and Cdc2
Abstract
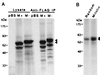
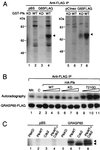
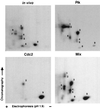
Cell division is characterized by orchestrated events of chromosome segregation, distribution of cellular organelles, and the eventual partitioning and separation of the two daughter cells. Mitotic kinases, including polo-like kinases (Plk), influence multiple events in mitosis. In yeast two-hybrid screens using mammalian Plk C-terminal domain baits, we have identified Golgi peripheral protein GRASP65 (Golgi reassembly stacking protein of 65 kDa) as a Plk-binding protein. GRASP65 appears to function in the postmitotic reassembly of Golgi stacks. In this report we demonstrate binding between Plk and GRASP65 and provide in vitro and in vivo evidence that Plk is a GRASP65 kinase. Moreover, we show that Cdc2 can also phosphorylate GRASP65. In addition, we present data which support the observation that the conserved C terminus of Plk is important for its function. Deletion or frameshift mutations in the conserved C-terminal domain of Plk greatly diminish its ability to phosphorylate GRASP65. These and previous findings suggest that phosphorylation of Golgi components by mitotic kinases may regulate mechanisms of Golgi inheritance during cell division.
Figures



Similar articles
-
Polo-like kinase is required for the fragmentation of pericentriolar Golgi stacks during mitosis.Proc Natl Acad Sci U S A. 2001 Jul 31;98(16):9128-32. doi: 10.1073/pnas.161283998. Epub 2001 Jul 10. Proc Natl Acad Sci U S A. 2001. PMID: 11447294 Free PMC article.
-
Plk1 docking to GRASP65 phosphorylated by Cdk1 suggests a mechanism for Golgi checkpoint signalling.EMBO J. 2005 Feb 23;24(4):753-65. doi: 10.1038/sj.emboj.7600569. Epub 2005 Jan 27. EMBO J. 2005. PMID: 15678101 Free PMC article.
-
Molecular mechanism of mitotic Golgi disassembly and reassembly revealed by a defined reconstitution assay.J Biol Chem. 2008 Mar 7;283(10):6085-94. doi: 10.1074/jbc.M707715200. Epub 2007 Dec 21. J Biol Chem. 2008. PMID: 18156178 Free PMC article.
-
The multiple facets of the Golgi reassembly stacking proteins.Biochem J. 2011 Feb 1;433(3):423-33. doi: 10.1042/BJ20101540. Biochem J. 2011. PMID: 21235525 Review.
-
In search of an essential step during mitotic Golgi disassembly and inheritance.Exp Cell Res. 2001 Nov 15;271(1):22-7. doi: 10.1006/excr.2001.5367. Exp Cell Res. 2001. PMID: 11697878 Review. No abstract available.
Cited by
-
Phosphorylation of Golgi Peripheral Membrane Protein Grasp65 Is an Integral Step in the Formation of the Human Cytomegalovirus Cytoplasmic Assembly Compartment.mBio. 2016 Oct 4;7(5):e01554-16. doi: 10.1128/mBio.01554-16. mBio. 2016. PMID: 27703074 Free PMC article.
-
Physical Association of Saccharomyces cerevisiae Polo-like Kinase Cdc5 with Chromosomal Cohesin Facilitates DNA Damage Response.J Biol Chem. 2016 Aug 12;291(33):17228-46. doi: 10.1074/jbc.M116.727438. Epub 2016 Jun 20. J Biol Chem. 2016. PMID: 27325700 Free PMC article.
-
Caspase cleavage of the Golgi stacking factor GRASP65 is required for Fas/CD95-mediated apoptosis.Cell Death Dis. 2010 Oct 7;1(10):e82. doi: 10.1038/cddis.2010.59. Cell Death Dis. 2010. PMID: 21368855 Free PMC article.
-
Phosphorylation provides a negative mode of regulation for the yeast Rab GTPase Sec4p.PLoS One. 2011;6(9):e24332. doi: 10.1371/journal.pone.0024332. Epub 2011 Sep 12. PLoS One. 2011. PMID: 21931684 Free PMC article.
-
GM130-dependent control of Cdc42 activity at the Golgi regulates centrosome organization.Mol Biol Cell. 2009 Feb;20(4):1192-200. doi: 10.1091/mbc.e08-08-0834. Epub 2008 Dec 24. Mol Biol Cell. 2009. PMID: 19109421 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

