Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion
- PMID: 11038187
- PMCID: PMC2192659
- DOI: 10.1083/jcb.151.2.413
Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion
Abstract
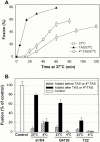
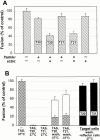
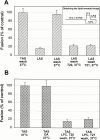
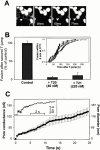
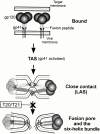
Many viral fusion proteins exhibit a six-helix bundle as a core structure. HIV Env-induced fusion was studied to resolve whether membrane merger was due to the transition into the bundle configuration or occurred after bundle formation. Suboptimal temperature was used to arrest fusion at an intermediate stage. When bundle formation was prevented by adding inhibitory peptides at this stage, membranes did not merge upon raising temperature. Inversely, when membrane merger was prevented by incorporating lysophosphatidylcholine (LPC) into cell membranes at the intermediate, the bundle did not form upon optimizing temperature. In the absence of LPC, the six-helix bundle did not form when the temperature of the intermediate was raised for times too short to promote fusion. Kinetic measures showed that after the temperature pulse, cells had not advanced further toward fusion. The latter results indicate that bundle formation is the rate-limiting step between the arrested intermediate and fusion. Electrical measures showed that the HIV Env-induced pore is initially large and grows rapidly. It is proposed that bundle formation and fusion are each contingent on the other and that movement of Env during its transition into the six-helix bundle directly induces the lipid rearrangements of membrane fusion. Because peptide inhibition showed that, at the intermediate stage, the heptad repeats of gp41 have become stably exposed, creation of the intermediate could be of importance in drug and/or vaccine development.
Figures
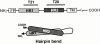

Comment in
-
HIV-1 membrane fusion: targets of opportunity.J Cell Biol. 2000 Oct 16;151(2):F9-14. doi: 10.1083/jcb.151.2.f9. J Cell Biol. 2000. PMID: 11038194 Free PMC article. No abstract available.
Similar articles
-
HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process.Biochemistry. 2001 Oct 16;40(41):12231-6. doi: 10.1021/bi0155596. Biochemistry. 2001. PMID: 11591141
-
Membrane-anchored inhibitory peptides capture human immunodeficiency virus type 1 gp41 conformations that engage the target membrane prior to fusion.J Virol. 2006 Apr;80(7):3249-58. doi: 10.1128/JVI.80.7.3249-3258.2006. J Virol. 2006. PMID: 16537592 Free PMC article.
-
HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation.Mol Biol Cell. 2003 Mar;14(3):926-38. doi: 10.1091/mbc.e02-09-0573. Mol Biol Cell. 2003. PMID: 12631714 Free PMC article.
-
The HIV Env-mediated fusion reaction.Biochim Biophys Acta. 2003 Jul 11;1614(1):36-50. doi: 10.1016/s0005-2736(03)00161-5. Biochim Biophys Acta. 2003. PMID: 12873764 Review.
-
Biochemistry and biophysics of HIV-1 gp41 - membrane interactions and implications for HIV-1 envelope protein mediated viral-cell fusion and fusion inhibitor design.Curr Top Med Chem. 2011 Dec;11(24):2959-84. doi: 10.2174/156802611798808497. Curr Top Med Chem. 2011. PMID: 22044229 Free PMC article. Review.
Cited by
-
RAGE inhibits human respiratory syncytial virus syncytium formation by interfering with F-protein function.J Gen Virol. 2013 Aug;94(Pt 8):1691-1700. doi: 10.1099/vir.0.049254-0. Epub 2013 Apr 4. J Gen Virol. 2013. PMID: 23559480 Free PMC article.
-
IFITM proteins restrict viral membrane hemifusion.PLoS Pathog. 2013 Jan;9(1):e1003124. doi: 10.1371/journal.ppat.1003124. Epub 2013 Jan 24. PLoS Pathog. 2013. PMID: 23358889 Free PMC article.
-
Distinct requirements for HIV-cell fusion and HIV-mediated cell-cell fusion.J Biol Chem. 2015 Mar 6;290(10):6558-73. doi: 10.1074/jbc.M114.623181. Epub 2015 Jan 14. J Biol Chem. 2015. PMID: 25589785 Free PMC article.
-
Release of gp120 Restraints Leads to an Entry-Competent Intermediate State of the HIV-1 Envelope Glycoproteins.mBio. 2016 Oct 25;7(5):e01598-16. doi: 10.1128/mBio.01598-16. mBio. 2016. PMID: 27795397 Free PMC article.
-
Ectodomain Pulling Combines with Fusion Peptide Inserting to Provide Cooperative Fusion for Influenza Virus and HIV.Int J Mol Sci. 2020 Jul 29;21(15):5411. doi: 10.3390/ijms21155411. Int J Mol Sci. 2020. PMID: 32751407 Free PMC article.
References
-
- Baker K.A., Dutch R.E., Lamb R.A., Jardetzky T.S. Structural basis for paramyxovirus-mediated membrane fusion. Mol. Cell. 1999;3:309–319. - PubMed
-
- Berger E.A., Murphy P.M., Farber J.M. Chemokine receptors as HIV-1 coreceptorsroles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. - PubMed
-
- Blacklow S.C., Lu M., Kim P.S. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry. 1995;34:14955–14962. - PubMed
-
- Chan D.C., Kim P.S. HIV entry and its inhibition. Cell. 1998;93:681–684. - PubMed
-
- Chan D.C., Fass D., Berger J.M., Kim P.S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

