Rapid transport of internalized P-selectin to late endosomes and the TGN: roles in regulating cell surface expression and recycling to secretory granules
- PMID: 11018057
- PMCID: PMC2189813
- DOI: 10.1083/jcb.151.1.107
Rapid transport of internalized P-selectin to late endosomes and the TGN: roles in regulating cell surface expression and recycling to secretory granules
Abstract
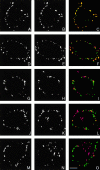
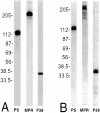
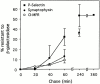
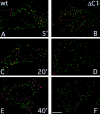
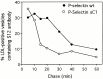
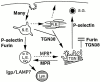
Prior studies on receptor recycling through late endosomes and the TGN have suggested that such traffic may be largely limited to specialized proteins that reside in these organelles. We present evidence that efficient recycling along this pathway is functionally important for nonresident proteins. P-selectin, a transmembrane cell adhesion protein involved in inflammation, is sorted from recycling cell surface receptors (e.g., low density lipoprotein [LDL] receptor) in endosomes, and is transported from the cell surface to the TGN with a half-time of 20-25 min, six to seven times faster than LDL receptor. Native P-selectin colocalizes with LDL, which is efficiently transported to lysosomes, for 20 min after internalization, but a deletion mutant deficient in endosomal sorting activity rapidly separates from the LDL pathway. Thus, P-selectin is sorted from LDL receptor in early endosomes, driving P-selectin rapidly into late endosomes. P-selectin then recycles to the TGN as efficiently as other receptors. Thus, the primary effect of early endosomal sorting of P-selectin is its rapid delivery to the TGN, with rapid turnover in lysosomes a secondary effect of frequent passage through late endosomes. This endosomal sorting event provides a mechanism for efficiently recycling secretory granule membrane proteins and, more generally, for downregulating cell surface receptors.
Figures
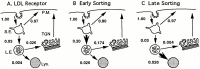
Similar articles
-
AP-3 adaptor functions in targeting P-selectin to secretory granules in endothelial cells.Traffic. 2001 Jun;2(6):406-13. doi: 10.1034/j.1600-0854.2001.002006406.x. Traffic. 2001. PMID: 11389768
-
Low density lipoprotein receptor and cation-independent mannose 6-phosphate receptor are transported from the cell surface to the Golgi apparatus at equal rates in PC12 cells.J Cell Biol. 1992 Apr;117(1):47-55. doi: 10.1083/jcb.117.1.47. J Cell Biol. 1992. PMID: 1313438 Free PMC article.
-
VAMP4 cycles from the cell surface to the trans-Golgi network via sorting and recycling endosomes.J Cell Sci. 2007 Mar 15;120(Pt 6):1028-41. doi: 10.1242/jcs.03387. Epub 2007 Feb 27. J Cell Sci. 2007. PMID: 17327277
-
Plant endosomes as protein sorting hubs.FEBS Lett. 2022 Sep;596(17):2288-2304. doi: 10.1002/1873-3468.14425. Epub 2022 Jun 17. FEBS Lett. 2022. PMID: 35689494 Review.
-
Retromer and sorting nexins in endosomal sorting.Biochem Soc Trans. 2015 Feb;43(1):33-47. doi: 10.1042/BST20140290. Biochem Soc Trans. 2015. PMID: 25619244 Review.
Cited by
-
Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network.Mol Biol Cell. 2004 Oct;15(10):4426-43. doi: 10.1091/mbc.e03-12-0872. Epub 2004 Jul 21. Mol Biol Cell. 2004. PMID: 15269279 Free PMC article.
-
Vascular targeting of nanocarriers: perplexing aspects of the seemingly straightforward paradigm.ACS Nano. 2014 May 27;8(5):4100-32. doi: 10.1021/nn500136z. Epub 2014 May 7. ACS Nano. 2014. PMID: 24787360 Free PMC article. Review.
-
Targeted endothelial nanomedicine for common acute pathological conditions.J Control Release. 2015 Dec 10;219:576-595. doi: 10.1016/j.jconrel.2015.09.055. Epub 2015 Oct 3. J Control Release. 2015. PMID: 26435455 Free PMC article. Review.
-
NLRP3 inflammasome activation in neutrophils directs early inflammatory response in murine peritonitis.Sci Rep. 2022 Dec 9;12(1):21313. doi: 10.1038/s41598-022-25176-4. Sci Rep. 2022. PMID: 36494392 Free PMC article.
-
Recycling of Raft-associated prohormone sorting receptor carboxypeptidase E requires interaction with ARF6.Mol Biol Cell. 2003 Nov;14(11):4448-57. doi: 10.1091/mbc.e02-11-0758. Epub 2003 Sep 5. Mol Biol Cell. 2003. PMID: 12960436 Free PMC article.
References
-
- Blagoveshchenskaya A.D., Norcott J.P., Cutler D.F. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic domain. J. Biol. Chem. 1998;273:2729–2737. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

