Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate
- PMID: 11003664
- PMCID: PMC86340
- DOI: 10.1128/MCB.20.20.7685-7692.2000
Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate
Abstract
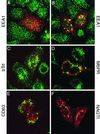
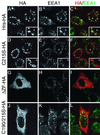
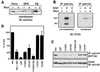
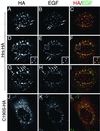
Hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) is a prominent substrate for activated tyrosine kinase receptors that has been proposed to play a role in endosomal membrane trafficking. The protein contains a FYVE domain, which specifically binds to the lipid phosphatidylinositol (PI) 3-phosphate (PI 3-P). We show that this interaction is required both for correct localization of the protein to endosomes that only partially coincides with early endosomal autoantigen 1 and for efficient tyrosine phosphorylation of the protein in response to epidermal growth factor stimulation. Treatment with wortmannin reveals that Hrs phosphorylation also requires PI 3-kinase activity, which is necessary to generate the PI 3-P required for localization. We have used both hypertonic media and expression of a dominant-negative form of dynamin (K44A) to inhibit endocytosis; under which conditions, receptor stimulation fails to elicit phosphorylation of Hrs. Our results provide a clear example of the coupling of a signal transduction pathway to endocytosis, from which we propose that activated receptor (or associated factor) must be delivered to the appropriate endocytic compartment in order for Hrs phosphorylation to occur.
Figures

Similar articles
-
Hrs recruits clathrin to early endosomes.EMBO J. 2001 Sep 3;20(17):5008-21. doi: 10.1093/emboj/20.17.5008. EMBO J. 2001. PMID: 11532964 Free PMC article.
-
The UIM domain of Hrs couples receptor sorting to vesicle formation.J Cell Sci. 2003 Oct 15;116(Pt 20):4169-79. doi: 10.1242/jcs.00723. Epub 2003 Sep 2. J Cell Sci. 2003. PMID: 12953068
-
The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signalling.Genes Cells. 2002 Mar;7(3):321-31. doi: 10.1046/j.1365-2443.2002.00519.x. Genes Cells. 2002. PMID: 11918675
-
Function of Hrs in endocytic trafficking and signalling.Biochem Soc Trans. 2001 Aug;29(Pt 4):472-5. doi: 10.1042/bst0290472. Biochem Soc Trans. 2001. PMID: 11498011 Review.
-
Hrs and endocytic sorting of ubiquitinated membrane proteins.Cell Struct Funct. 2002 Dec;27(6):403-8. doi: 10.1247/csf.27.403. Cell Struct Funct. 2002. PMID: 12576633 Review.
Cited by
-
Endofin is required for HD-PTP and ESCRT-0 interdependent endosomal sorting of ubiquitinated transmembrane cargoes.iScience. 2021 Oct 14;24(11):103274. doi: 10.1016/j.isci.2021.103274. eCollection 2021 Nov 19. iScience. 2021. PMID: 34761192 Free PMC article.
-
Motor and Sensory Deficits in the teetering Mice Result from Mutation of the ESCRT Component HGS.PLoS Genet. 2015 Jun 26;11(6):e1005290. doi: 10.1371/journal.pgen.1005290. eCollection 2015 Jun. PLoS Genet. 2015. PMID: 26115514 Free PMC article.
-
Mutation of SIMPLE in Charcot-Marie-Tooth 1C alters production of exosomes.Mol Biol Cell. 2013 Jun;24(11):1619-37, S1-3. doi: 10.1091/mbc.E12-07-0544. Epub 2013 Apr 10. Mol Biol Cell. 2013. PMID: 23576546 Free PMC article.
-
Hepatocyte growth factor-regulated tyrosine kinase substrate (HGS) and guanylate kinase 1 (GUK1) are differentially expressed in GH-secreting adenomas.Pituitary. 2006;9(2):83-92. doi: 10.1007/s11102-006-9277-1. Pituitary. 2006. PMID: 16832584
-
Regulation of EGFR signal transduction by analogue-to-digital conversion in endosomes.Elife. 2015 Feb 4;4:e06156. doi: 10.7554/eLife.06156. Elife. 2015. PMID: 25650738 Free PMC article.
References
-
- Asao H, Sasaki Y, Arita T, Tanaka N, Endo K, Kasai H, Takeshita T, Endo Y, Fujita T, Sugamura K. Hrs is associated with STAM, a signal-transducing adaptor molecule. J Biol Chem. 1997;272:32785–32791. - PubMed
-
- Bean A J, Seifert R, Chen Y A, Sacks R, Scheller R H. Hrs-2 is an ATPase implicated in calcium-regulated secretion. Nature. 1997;385:826–829. - PubMed
-
- Burd C G, Babst M, Emr S D. Novel pathways, membrane coats and PI kinase regulation in yeast lysosomal trafficking. Semin Cell Dev Biol. 1998;9:527–533. - PubMed
-
- Burd C G, Emr S D. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol Cell. 1998;2:157–162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
