Identification of sites phosphorylated by the vaccinia virus B1R kinase in viral protein H5R
- PMID: 11001589
- PMCID: PMC29058
- DOI: 10.1186/1471-2091-1-2
Identification of sites phosphorylated by the vaccinia virus B1R kinase in viral protein H5R
Abstract
Background: Vaccinia virus gene B1R encodes a serine/threonine protein kinase. In vitro this protein kinase phosphorylates ribosomal proteins Sa and S2 and vaccinia virus protein H5R, proteins that become phosphorylated during infection. Nothing is known about the sites phosphorylated on these proteins or the general substrate specificity of the kinase. The work described is the first to address these questions.
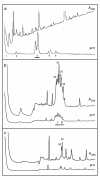
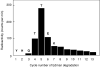
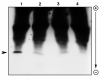
Results: Vaccinia virus protein H5R was phosphorylated by the B1R protein kinase in vitro, digested with V8 protease, and phosphopeptides separated by HPLC. The N-terminal sequence of one radioactively labelled phosphopeptide was determined and found to correspond to residues 81-87 of the protein, with Thr-84 and Thr-85 being phosphorylated. A synthetic peptide based on this region of the protein was shown to be a substrate for the B1R protein kinase, and the extent of phosphorylation was substantially decreased if either Thr residue was replaced by an Ala.
Conclusions: We have identified the first phosphorylation site for the vaccinia virus B1R protein kinase. This gives important information about the substrate-specificity of the enzyme, which differs from that of other known protein kinases. It remains to be seen whether the same site is phosphorylated in vivo.
Figures
Similar articles
-
Vaccinia virus gene H5R encodes a protein that is phosphorylated by the multisubstrate vaccinia virus B1R protein kinase.J Virol. 1995 Mar;69(3):1819-26. doi: 10.1128/JVI.69.3.1819-1826.1995. J Virol. 1995. PMID: 7853522 Free PMC article.
-
Vaccinia virus B1R kinase interacts with JIP1 and modulates c-Jun-dependent signaling.J Virol. 2006 Aug;80(15):7667-75. doi: 10.1128/JVI.00967-06. J Virol. 2006. PMID: 16840345 Free PMC article.
-
Temperature-dependent phosphorylation state of the H5R protein synthesised at the early stage of infection in cells infected with vaccinia virus ts mutants of the B1R and F10L protein kinases.Intervirology. 2000;43(1):67-70. doi: 10.1159/000025025. Intervirology. 2000. PMID: 10773740
-
Emerging biological functions of the vaccinia-related kinase (VRK) family.Histol Histopathol. 2009 Jun;24(6):749-59. doi: 10.14670/HH-24.749. Histol Histopathol. 2009. PMID: 19337973 Review.
-
Posttranslational modification of vaccinia virus proteins.Curr Top Microbiol Immunol. 1990;163:185-206. doi: 10.1007/978-3-642-75605-4_7. Curr Top Microbiol Immunol. 1990. PMID: 2242680 Review. No abstract available.
Cited by
-
A poxvirus pseudokinase represses viral DNA replication via a pathway antagonized by its paralog kinase.PLoS Pathog. 2019 Feb 15;15(2):e1007608. doi: 10.1371/journal.ppat.1007608. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30768651 Free PMC article.
-
Orthopoxvirus targets for the development of antiviral therapies.Curr Drug Targets Infect Disord. 2005 Mar;5(1):17-28. doi: 10.2174/1568005053174627. Curr Drug Targets Infect Disord. 2005. PMID: 15777195 Free PMC article. Review.
-
The vaccinia-related kinases phosphorylate the N' terminus of BAF, regulating its interaction with DNA and its retention in the nucleus.Mol Biol Cell. 2006 May;17(5):2451-64. doi: 10.1091/mbc.e05-12-1179. Epub 2006 Feb 22. Mol Biol Cell. 2006. PMID: 16495336 Free PMC article.
-
Clustered charge-to-alanine mutagenesis of the vaccinia virus A20 gene: temperature-sensitive mutants have a DNA-minus phenotype and are defective in the production of processive DNA polymerase activity.J Virol. 2001 Dec;75(24):12308-18. doi: 10.1128/JVI.75.24.12308-12318.2001. J Virol. 2001. PMID: 11711621 Free PMC article.
-
Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles.J Virol. 2006 Mar;80(5):2127-40. doi: 10.1128/JVI.80.5.2127-2140.2006. J Virol. 2006. PMID: 16474121 Free PMC article.
References
-
- Moss B. Poxviridae: the viruses and their replication. In Fields Virology, 3rd edn (Edited by Field BN, Knipe DM, Howley, PM) Philadelphia: Lippincott-Raven, 1996. pp. 2637–2671.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

