Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons
- PMID: 10995834
- PMCID: PMC6772823
- DOI: 10.1523/JNEUROSCI.20-18-06898.2000
Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons
Abstract
Cathepsin D-deficient (CD-/-) mice have been shown to manifest seizures and become blind near the terminal stage [approximately postnatal day (P) 26]. We therefore examined the morphological, immunocytochemical, and biochemical features of CNS tissues of these mice. By electron microscopy, autophagosome/autolysosome-like bodies containing part of the cytoplasm, granular osmiophilic deposits, and fingerprint profiles were demonstrated in the neuronal perikarya of CD-/- mouse brains after P20. Autophagosomes and granular osmiophilic deposits were detected in neurons at P0 but were few in number, whereas they increased in the neuronal perikarya within days after birth. Some large-sized neurons having autophagosome/autolysosome-like bodies in the perikarya appeared in the CNS tissues, especially in the thalamic region and the cerebral cortex, at P17. These lysosomal bodies occupied the perikarya of almost all neurons in CD-/- mouse brains obtained from P23 until the terminal stage. Because these neurons exhibited autofluorescence, it was considered that ceroid lipofuscin may accumulate in lysosomal structures of CD-/- neurons. Subunit c of mitochondrial ATP synthase was found to accumulate in the lysosomes of neurons, although the activity of tripeptidyl peptidase-I significantly increased in the brain. Moreover, neurons near the terminal stage were often shrunken and possessed irregular nuclei through which small dense chromatin masses were scattered. These results suggest that the CNS neurons in CD-/- mice show a new form of lysosomal accumulation disease with a phenotype resembling neuronal ceroid lipofuscinosis.
Figures

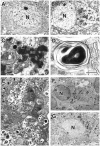
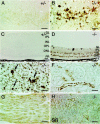
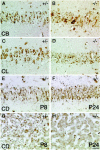
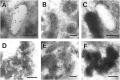
Similar articles
-
Participation of autophagy in storage of lysosomes in neurons from mouse models of neuronal ceroid-lipofuscinoses (Batten disease).Am J Pathol. 2005 Dec;167(6):1713-28. doi: 10.1016/S0002-9440(10)61253-9. Am J Pathol. 2005. PMID: 16314482 Free PMC article.
-
Enzyme replacement therapy with recombinant pro-CTSD (cathepsin D) corrects defective proteolysis and autophagy in neuronal ceroid lipofuscinosis.Autophagy. 2020 May;16(5):811-825. doi: 10.1080/15548627.2019.1637200. Epub 2019 Jul 16. Autophagy. 2020. PMID: 31282275 Free PMC article.
-
Involvement of nitric oxide released from microglia-macrophages in pathological changes of cathepsin D-deficient mice.J Neurosci. 2001 Oct 1;21(19):7526-33. doi: 10.1523/JNEUROSCI.21-19-07526.2001. J Neurosci. 2001. PMID: 11567042 Free PMC article.
-
Lysosomal storage of the DCCD reactive proteolipid subunit of mitochondrial ATP synthase in human and ovine ceroid lipofuscinoses.Adv Exp Med Biol. 1989;266:211-22; discussion 223. doi: 10.1007/978-1-4899-5339-1_15. Adv Exp Med Biol. 1989. PMID: 2535017 Review.
-
Developmental changes in the expression of neuronal ceroid lipofuscinoses-linked proteins.Mol Genet Metab. 2000 Sep-Oct;71(1-2):190-4. doi: 10.1006/mgme.2000.3071. Mol Genet Metab. 2000. PMID: 11001810 Review.
Cited by
-
Microglial functions and proteases.Mol Neurobiol. 2003 Apr;27(2):163-76. doi: 10.1385/MN:27:2:163. Mol Neurobiol. 2003. PMID: 12777686 Review.
-
Rab6 promotes insulin receptor and cathepsin trafficking to regulate autophagy induction and activity in Drosophila.J Cell Sci. 2018 Sep 7;131(17):jcs216127. doi: 10.1242/jcs.216127. J Cell Sci. 2018. PMID: 30111579 Free PMC article.
-
Cathepsin deficiency as a model for neuronal ceroid lipofuscinoses.Am J Pathol. 2005 Dec;167(6):1473-6. doi: 10.1016/S0002-9440(10)61233-3. Am J Pathol. 2005. PMID: 16314462 Free PMC article. Review. No abstract available.
-
[NCL in animal models].Ophthalmologe. 2010 Jul;107(7):621-7. doi: 10.1007/s00347-009-2108-9. Ophthalmologe. 2010. PMID: 20454900 German.
-
Structural basis for the wobbler mouse neurodegenerative disorder caused by mutation in the Vps54 subunit of the GARP complex.Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):12860-5. doi: 10.1073/pnas.1004756107. Epub 2010 Jul 6. Proc Natl Acad Sci U S A. 2010. PMID: 20615984 Free PMC article.
References
-
- Banay-Schwartz M, Bracco F, DeGuzman T, Lajtha A. Developmental changes in the breakdown of brain tubulin by cerebral cathepsin D. Neurochem Res. 1983;8:51–61. - PubMed
-
- Banay-Schwartz M, Dahl D, Hui KS, Lajtha A. The breakdown of the individual neurofilament proteins by cathepsin D. Neurochem Res. 1987;12:361–367. - PubMed
-
- Bando Y, Kominami E, Katunuma N. Purification and tissue distribution of rat cathepsin L. J Biochem (Tokyo) 1986;100:35–42. - PubMed
-
- Barrett AJ, Kirsche H. Cathepsin B, cathepsin H, cathepsin L. Methods Enzymol. 1981;80:535–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
