Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen
- PMID: 10995787
- PMCID: PMC381391
- DOI: 10.1172/JCI9180
Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen
Abstract
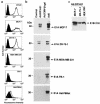
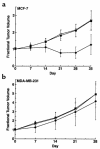
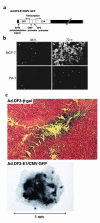
The DF3/MUC1 gene is aberrantly overexpressed in human breast and other carcinomas. Previous studies have demonstrated that the DF3/MUC1 promoter/enhancer confers selective expression of diverse transgenes in MUC1-positive breast cancer cells. In this study, we show that an adenoviral vector (Ad.DF3-E1) in which the DF3/MUC1 promoter drives expression of E1A selectively replicates in MUC1-positive breast cancer cells. We also show that Ad.DF3-E1 infection of human breast tumor xenografts in nude mice is associated with inhibition of tumor growth. In contrast to a replication-incompetent adenoviral vector that infects along the injection track, Ad.DF3-E1 infection was detectable throughout the tumor xenografts. To generate an Ad.DF3-E1 vector with the capacity for incorporating therapeutic products, we inserted the cytomegalovirus (CMV) promoter upstream of the TNF cDNA. Infection with Ad.DF3-E1/CMV-TNF was associated with selective replication and production of TNF in cells that express MUC1. Moreover, treatment of MUC1-positive, but not MUC1-negative, xenografts with a single injection of Ad.DF3-E1/CMV-TNF was effective in inducing stable tumor regression. These findings demonstrate that the DF3/MUC1 promoter confers competence for selective replication of Ad.DF3-E1 in MUC1-positive breast tumor cells, and that the antitumor activity of this vector is potentiated by integration of the TNF cDNA.
Figures




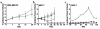
Similar articles
-
Dual E1A oncolytic adenovirus: targeting tumor heterogeneity with two independent cancer-specific promoter elements, DF3/MUC1 and hTERT.Cancer Gene Ther. 2011 Mar;18(3):153-66. doi: 10.1038/cgt.2010.52. Epub 2010 Sep 24. Cancer Gene Ther. 2011. PMID: 20865021 Free PMC article.
-
Selective gene expression using a DF3/MUC1 promoter in a human esophageal adenocarcinoma model.Gene Ther. 2003 Feb;10(3):206-12. doi: 10.1038/sj.gt.3301867. Gene Ther. 2003. PMID: 12571627
-
Breast cancer selective gene expression and therapy mediated by recombinant adenoviruses containing the DF3/MUC1 promoter.J Clin Invest. 1995 Dec;96(6):2775-82. doi: 10.1172/JCI118347. J Clin Invest. 1995. PMID: 8675647 Free PMC article.
-
Selectivity of an oncolytic herpes simplex virus for cells expressing the DF3/MUC1 antigen.Cancer Res. 2004 Apr 1;64(7):2561-7. doi: 10.1158/0008-5472.can-03-3431. Cancer Res. 2004. PMID: 15059912
-
Hormonal regulation of MUC1 expression.Int J Biol Markers. 1999 Jan-Mar;14(1):29-35. doi: 10.1177/172460089901400106. Int J Biol Markers. 1999. PMID: 10367247 Review.
Cited by
-
Enhancing the therapeutic efficacy of adenovirus in combination with biomaterials.Biomaterials. 2012 Feb;33(6):1838-50. doi: 10.1016/j.biomaterials.2011.11.020. Epub 2011 Dec 3. Biomaterials. 2012. PMID: 22142769 Free PMC article. Review.
-
Dual E1A oncolytic adenovirus: targeting tumor heterogeneity with two independent cancer-specific promoter elements, DF3/MUC1 and hTERT.Cancer Gene Ther. 2011 Mar;18(3):153-66. doi: 10.1038/cgt.2010.52. Epub 2010 Sep 24. Cancer Gene Ther. 2011. PMID: 20865021 Free PMC article.
-
Regulation of herpes simplex virus 1 replication using tumor-associated promoters.Ann Surg. 2002 Oct;236(4):502-12; discussion 512-3. doi: 10.1097/00000658-200210000-00013. Ann Surg. 2002. PMID: 12368679 Free PMC article.
-
Potent antitumoral efficacy of a novel replicative adenovirus CNHK300 targeting telomerase-positive cancer cells.J Cancer Res Clin Oncol. 2004 Oct;130(10):591-603. doi: 10.1007/s00432-004-0577-4. Epub 2004 Jul 9. J Cancer Res Clin Oncol. 2004. PMID: 15243805
-
A uniquely stable replication-competent retrovirus vector achieves efficient gene delivery in vitro and in solid tumors.Hum Gene Ther. 2001 May 20;12(8):921-32. doi: 10.1089/104303401750195881. Hum Gene Ther. 2001. PMID: 11387057 Free PMC article.
References
-
- Haj-Ahmad Y, Graham F. Characterization of an adenovirus type 5 mutant carrying embedded inverted terminal repeats. Virology. 1986;153:22–34. - PubMed
-
- Rodriguez R, et al. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. - PubMed
-
- Miyatake S-I, et al. Hepatoma-specific antitumor activity of an albumin enhancer/promoter regulated herpes simplex virus in vivo. Gene Ther. 1999;6:564–572. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

