Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription
- PMID: 10990461
- PMCID: PMC314216
- DOI: 10.1093/emboj/19.18.4976
Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription
Abstract
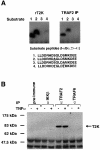
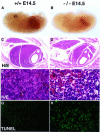
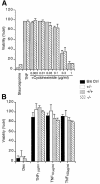
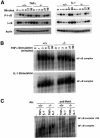
Induction of NF-kappaB-dependent transcription requires phosphorylation and subsequent degradation of I-kappaB, an inhibitor of NF-kappaB, followed by nuclear translocation and DNA binding of NF-kappaB. Tumor necrosis factor receptor-associated factor 2 (TRAF2) plays a role in NF-kappaB activation in response to cytokines such as tumor necrosis factor alpha (TNFalpha). In this study, we purified and characterized a novel kinase (T2K, also known as TBK1 or NAK), which associates with TRAF2 and exhibits kinase activity towards I-kappaBalpha in vitro. The physiological function of T2K was investigated using T2K-deficient mice. Heterozygotes appear normal, but t2k(-/-) animals die at approximately E14.5 of massive liver degeneration and apoptosis. Never theless, hematopoietic progenitors from T2K-deficient fetal liver support normal lymphocyte development. Furthermore, t2k(-/-) embryonic fibroblasts and thymocytes do not display increased sensitivity to TNFalpha-induced apoptosis. In response to either TNFalpha or IL-1 induction, t2k(-/-) embryonic fibroblasts exhibit normal degradation of I-kappaB and kappaB-binding activity. However, NF-kappaB-directed transcription is dramatically reduced. These results demonstrate that, like I-kappaB kinase beta and the RelA subunit of NF-kappaB, T2K is critical in protecting embryonic liver from apoptosis. However, T2K has a unique role in the activation of NF-kappaB-directed transcription, apparently independent of I-kappaB degradation and NF-kappaB DNA binding.
Figures

Similar articles
-
Regulation and function of IKK and IKK-related kinases.Sci STKE. 2006 Oct 17;2006(357):re13. doi: 10.1126/stke.3572006re13. Sci STKE. 2006. PMID: 17047224 Review.
-
Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice.Am J Pathol. 2000 Mar;156(3):997-1007. doi: 10.1016/S0002-9440(10)64967-X. Am J Pathol. 2000. PMID: 10702415 Free PMC article.
-
Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression.Mol Cell Biol. 2006 Apr;26(8):3071-84. doi: 10.1128/MCB.26.8.3071-3084.2006. Mol Cell Biol. 2006. PMID: 16581782 Free PMC article.
-
Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway.Mol Cell. 2001 Oct;8(4):771-80. doi: 10.1016/s1097-2765(01)00361-6. Mol Cell. 2001. PMID: 11684013
-
NF-kappaB activation and HIV-1 induced apoptosis.Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):235-53. doi: 10.1016/s1359-6101(99)00015-5. Cytokine Growth Factor Rev. 1999. PMID: 10647779 Review.
Cited by
-
A TRAF-like motif of the inducible costimulator ICOS controls development of germinal center TFH cells via the kinase TBK1.Nat Immunol. 2016 Jul;17(7):825-33. doi: 10.1038/ni.3463. Epub 2016 May 2. Nat Immunol. 2016. PMID: 27135603 Free PMC article.
-
Stress granules at the intersection of autophagy and ALS.Brain Res. 2016 Oct 15;1649(Pt B):189-200. doi: 10.1016/j.brainres.2016.05.022. Epub 2016 May 13. Brain Res. 2016. PMID: 27181519 Free PMC article. Review.
-
TNFR1 signaling kinetics: spatiotemporal control of three phases of IKK activation by posttranslational modification.Cell Signal. 2013 Aug;25(8):1654-64. doi: 10.1016/j.cellsig.2013.04.005. Epub 2013 Apr 21. Cell Signal. 2013. PMID: 23612498 Free PMC article. Review.
-
The Type I Interferon-IRF7 Axis Mediates Transcriptional Expression of Usp25 Gene.J Biol Chem. 2016 Jun 17;291(25):13206-15. doi: 10.1074/jbc.M116.718080. Epub 2016 Apr 27. J Biol Chem. 2016. PMID: 27129230 Free PMC article.
-
S6K-STING interaction regulates cytosolic DNA-mediated activation of the transcription factor IRF3.Nat Immunol. 2016 May;17(5):514-522. doi: 10.1038/ni.3433. Epub 2016 Apr 4. Nat Immunol. 2016. PMID: 27043414 Free PMC article.
References
-
- Amakawa R. et al. (1996) Impaired negative selection of T cells in Hodgkin’s disease antigen CD30-deficient mice. Cell, 84, 551–562. - PubMed
-
- Anrather J., Csizmadia,V., Soares,M.P. and Winkler,H. (1999) Regulation of NF-κB RelA phosphorylation and transcriptional activity by p21(ras) and protein kinase Cζ in primary endothelial cells. J. Biol. Chem., 274, 13594–13603. - PubMed
-
- Arch R.H., Gedrich,R.W. and Thompson,C.B. (1998) Tumor necrosis factor receptor-associated factors (TRAFs)—a family of adapter proteins that regulates life and death. Genes Dev., 12, 2821–2830. - PubMed
-
- Beg A.A. and Baltimore,D. (1996) An essential role for NF-κB in preventing TNF-α-induced cell death. Science, 274, 782–784. - PubMed
-
- Beg A.A., Sha,W.C., Bronson,R.T., Ghosh,S. and Baltimore,D. (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kB. Nature, 376, 167–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

