The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling
- PMID: 10990458
- PMCID: PMC314225
- DOI: 10.1093/emboj/19.18.4944
The C-terminal cytoplasmic Lys-thr-X-X-X-Trp motif in frizzled receptors mediates Wnt/beta-catenin signalling
Abstract
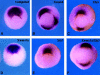
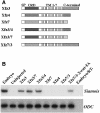
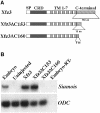
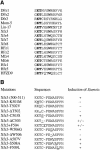
Frizzled receptors are components of the Wnt signalling pathway, but how they activate the canonical Wnt/beta-catenin pathway is not clear. Here we use three distinct vertebrate frizzled receptors (Xfz3, Xfz4 and Xfz7) and describe whether and how their C-terminal cytoplasmic regions transduce the Wnt/beta-catenin signal. We show that Xfz3 activates this pathway in the absence of exogenous ligands, while Xfz4 and Xfz7 interact with Xwnt5A to activate this pathway. Analysis using chimeric receptors reveals that their C-terminal cytoplasmic regions are functionally equivalent in Wnt/beta-catenin signalling. Furthermore, a conserved motif (Lys-Thr-X-X-X-Trp) located two amino acids after the seventh transmembrane domain is required for activation of the Wnt/beta-catenin pathway and for membrane relocalization and phosphorylation of Dishevelled. Frizzled receptors with point mutations affecting either of the three conserved residues are defective in Wnt/beta-catenin signalling. These findings provide functional evidence supporting a role of this conserved motif in the modulation of Wnt signalling. They are consistent with the genetic features exhibited by Drosophila Dfz3 and Caenorhabditis elegans mom-5 in which the tryptophan is substituted by a tyrosine.
Figures


Similar articles
-
Frizzled receptor dimerization is sufficient to activate the Wnt/beta-catenin pathway.J Cell Sci. 2003 Jun 15;116(Pt 12):2541-50. doi: 10.1242/jcs.00451. Epub 2003 May 6. J Cell Sci. 2003. PMID: 12734397
-
Interaction of Frizzled 7 and Dishevelled in Xenopus.Dev Dyn. 2000 Aug;218(4):671-80. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1017>3.0.CO;2-9. Dev Dyn. 2000. PMID: 10906785
-
The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt. beta-catenin signaling cascade.J Biol Chem. 2004 Apr 9;279(15):14879-88. doi: 10.1074/jbc.M306421200. Epub 2004 Jan 27. J Biol Chem. 2004. PMID: 14747478
-
New steps in the Wnt/beta-catenin signal transduction pathway.Recent Prog Horm Res. 2000;55:225-36. Recent Prog Horm Res. 2000. PMID: 11036939 Review.
-
Modulation of Wnt signaling by Axin and Axil.Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review.
Cited by
-
Genome-Wide Association Study of Down Syndrome-Associated Atrioventricular Septal Defects.G3 (Bethesda). 2015 Jul 20;5(10):1961-71. doi: 10.1534/g3.115.019943. G3 (Bethesda). 2015. PMID: 26194203 Free PMC article.
-
GSK-3 activity is critical for the orientation of the cortical microtubules and the dorsoventral axis determination in zebrafish embryos.PLoS One. 2012;7(5):e36655. doi: 10.1371/journal.pone.0036655. Epub 2012 May 4. PLoS One. 2012. PMID: 22574208 Free PMC article.
-
Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt.EMBO J. 2010 Jul 7;29(13):2114-25. doi: 10.1038/emboj.2010.100. Epub 2010 May 21. EMBO J. 2010. PMID: 20495530 Free PMC article.
-
Role of PDZ proteins in regulating trafficking, signaling, and function of GPCRs: means, motif, and opportunity.Adv Pharmacol. 2011;62:279-314. doi: 10.1016/B978-0-12-385952-5.00003-8. Adv Pharmacol. 2011. PMID: 21907913 Free PMC article. Review.
-
Combinatorial Wnt signaling landscape during brachiopod anteroposterior patterning.BMC Biol. 2024 Sep 19;22(1):212. doi: 10.1186/s12915-024-01988-w. BMC Biol. 2024. PMID: 39300453 Free PMC article.
References
-
- Adler P.N. (1992) The genetic control of tissue polarity in Drosophila. BioEssays, 14, 735–741. - PubMed
-
- Bassez T., Paris,J., Omilli,F., Dorel,C. and Osborne,H.B. (1990) Post-transcriptional regulation of ornithine decarboxylase in Xenopus laevis. Development, 110, 955–962. - PubMed
-
- Bhanot P., Brink,M., Samos,C.H., Hsieh,J.C., Wang,Y.S., Macke,J.P., Andrew,D., Nathans,J. and Nusse,R. (1996) A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature, 382, 225–230. - PubMed
-
- Bhanot P., Fish,M., Jemison,J.A., Nusse,R., Nathans,J. and Cadigan,K.M. (1999) Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development, 126, 4175–4186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

