Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and Is required for late endosome-lysosome fusion
- PMID: 10982406
- PMCID: PMC14981
- DOI: 10.1091/mbc.11.9.3137
Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and Is required for late endosome-lysosome fusion
Abstract
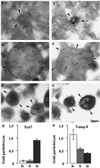
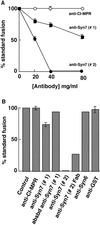
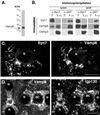
Protein traffic from the cell surface or the trans-Golgi network reaches the lysosome via a series of endosomal compartments. One of the last steps in the endocytic pathway is the fusion of late endosomes with lysosomes. This process has been reconstituted in vitro and has been shown to require NSF, alpha and gamma SNAP, and a Rab GTPase based on inhibition by Rab GDI. In Saccharomyces cerevisiae, fusion events to the lysosome-like vacuole are mediated by the syntaxin protein Vam3p, which is localized to the vacuolar membrane. In an effort to identify the molecular machinery that controls fusion events to the lysosome, we searched for mammalian homologues of Vam3p. One such candidate is syntaxin 7. Here we show that syntaxin 7 is concentrated in late endosomes and lysosomes. Coimmunoprecipitation experiments show that syntaxin 7 is associated with the endosomal v-SNARE Vamp 8, which partially colocalizes with syntaxin 7. Importantly, we show that syntaxin 7 is specifically required for the fusion of late endosomes with lysosomes in vitro, resulting in a hybrid organelle. Together, these data identify a SNARE complex that functions in the late endocytic system of animal cells.
Figures
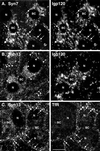
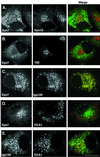
Similar articles
-
Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages.Mol Biol Cell. 2000 Jul;11(7):2327-33. doi: 10.1091/mbc.11.7.2327. Mol Biol Cell. 2000. PMID: 10888671 Free PMC article.
-
The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes.Mol Biol Cell. 2000 Oct;11(10):3289-98. doi: 10.1091/mbc.11.10.3289. Mol Biol Cell. 2000. PMID: 11029036 Free PMC article.
-
Endosome-lysosome fusion.Biochem Soc Trans. 2010 Dec;38(6):1413-6. doi: 10.1042/BST0381413. Biochem Soc Trans. 2010. PMID: 21118098
-
Relationship between endosomes and lysosomes.Biochem Soc Trans. 2001 Aug;29(Pt 4):476-80. doi: 10.1042/bst0290476. Biochem Soc Trans. 2001. PMID: 11498012 Review.
-
The delivery of endocytosed cargo to lysosomes.Biochem Soc Trans. 2009 Oct;37(Pt 5):1019-21. doi: 10.1042/BST0371019. Biochem Soc Trans. 2009. PMID: 19754443 Review.
Cited by
-
Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes.Mol Biol Cell. 2002 Apr;13(4):1313-28. doi: 10.1091/mbc.01-10-0525. Mol Biol Cell. 2002. PMID: 11950941 Free PMC article.
-
Mammalian ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain.Mol Biol Cell. 2003 Feb;14(2):698-720. doi: 10.1091/mbc.e02-09-0556. Mol Biol Cell. 2003. PMID: 12589064 Free PMC article.
-
ESCRT-I function is required for Tyrp1 transport from early endosomes to the melanosome limiting membrane.Traffic. 2009 Sep;10(9):1318-36. doi: 10.1111/j.1600-0854.2009.00955.x. Epub 2009 Jun 9. Traffic. 2009. PMID: 19624486 Free PMC article.
-
Involvement of vesicle-associated membrane protein 7 in human gastric epithelial cell vacuolation induced by Helicobacter pylori-produced VacA.Infect Immun. 2008 Jun;76(6):2296-303. doi: 10.1128/IAI.01573-07. Epub 2008 Mar 24. Infect Immun. 2008. PMID: 18362137 Free PMC article.
-
Get Closer to the World of Contact Sites: A Beginner's Guide to Proximity-Driven Fluorescent Probes.Contact (Thousand Oaks). 2022 Dec 15;5:25152564221135748. doi: 10.1177/25152564221135748. eCollection 2022 Jan-Dec. Contact (Thousand Oaks). 2022. PMID: 37366505 Free PMC article. Review.
References
-
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem. 1998;273:10317–10324. - PubMed
-
- Agard DA, Yasushi H, Shaw P, Sedat JW. Fluorescence microscopy in three dimensions. Methods Cell Biol. 1989;30:353–377. - PubMed
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

