Fas ligand, Bcl-2, granulocyte colony-stimulating factor, and p38 mitogen-activated protein kinase: Regulators of distinct cell death and survival pathways in granulocytes
- PMID: 10974031
- PMCID: PMC2193264
- DOI: 10.1084/jem.192.5.647
Fas ligand, Bcl-2, granulocyte colony-stimulating factor, and p38 mitogen-activated protein kinase: Regulators of distinct cell death and survival pathways in granulocytes
Abstract
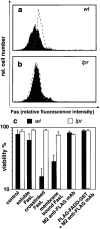
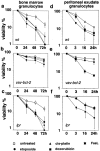
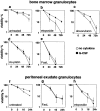
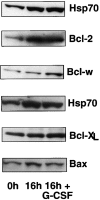
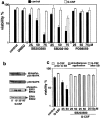
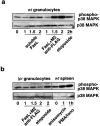
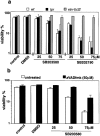
The short life span of granulocytes, which limits many inflammatory responses, is thought to be influenced by the Bcl-2 protein family, death receptors such as CD95 (Fas/APO-1), stress-activated protein kinases such as p38 mitogen-activated protein kinase (MAPK), and proinflammatory cytokines like granulocyte colony-stimulating factor (G-CSF). To clarify the roles of these various regulators in granulocyte survival, we have investigated the spontaneous apoptosis of granulocytes in culture and that induced by Fas ligand or chemotherapeutic drugs, using cells from normal, CD95-deficient lpr, or vav-bcl-2 transgenic mice. CD95-induced apoptosis, which required receptor aggregation by recombinant Fas ligand or the membrane-bound ligand, was unaffected by G-CSF treatment or Bcl-2 overexpression. Conversely, spontaneous and drug-induced apoptosis occurred normally in lpr granulocytes but were suppressed by G-CSF treatment or Bcl-2 overexpression. Although activation of p38 MAPK has been implicated in granulocyte death, their apoptosis actually was markedly accelerated by specific inhibitors of this kinase. These results suggest that G-CSF promotes granulocyte survival largely through the Bcl-2-controlled pathway, whereas CD95 regulates a distinct pathway to apoptosis that is not required for either their spontaneous or drug-induced death. Moreover, p38 MAPK signaling contributes to granulocyte survival rather than their apoptosis.
Figures

Comment in
-
Cross-talk in cell death signaling.J Exp Med. 2000 Oct 16;192(8):F21-5. J Exp Med. 2000. PMID: 11034612 Free PMC article. Review. No abstract available.
Similar articles
-
Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis.J Immunol. 2002 Apr 15;168(8):4025-33. doi: 10.4049/jimmunol.168.8.4025. J Immunol. 2002. PMID: 11937560
-
Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival.Blood. 2003 Mar 15;101(6):2393-400. doi: 10.1182/blood-2002-07-2132. Epub 2002 Nov 14. Blood. 2003. PMID: 12433687
-
Extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways cooperate in mediating cytokine-induced proliferation of a leukemic cell line.Leukemia. 2002 Feb;16(2):244-53. doi: 10.1038/sj.leu.2402367. Leukemia. 2002. PMID: 11840291
-
Regulation of eosinophil and neutrophil apoptosis--similarities and differences.Immunol Rev. 2001 Feb;179:156-62. doi: 10.1034/j.1600-065x.2001.790115.x. Immunol Rev. 2001. PMID: 11292018 Review.
-
Activation of mitogen-activated protein kinase pathways by the granulocyte colony-stimulating factor receptor: mechanisms and functional consequences.Front Biosci. 2007 Jan 1;12:591-607. doi: 10.2741/2085. Front Biosci. 2007. PMID: 17127320 Review.
Cited by
-
Eosinophils in the lung - modulating apoptosis and efferocytosis in airway inflammation.Front Immunol. 2014 Jul 1;5:302. doi: 10.3389/fimmu.2014.00302. eCollection 2014. Front Immunol. 2014. PMID: 25071763 Free PMC article. Review.
-
NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP.J Cell Biol. 2004 Aug 2;166(3):369-80. doi: 10.1083/jcb.200401036. J Cell Biol. 2004. PMID: 15289496 Free PMC article.
-
Overexpression of Mcl-1 exacerbates lymphocyte accumulation and autoimmune kidney disease in lpr mice.Cell Death Differ. 2017 Mar;24(3):397-408. doi: 10.1038/cdd.2016.125. Epub 2016 Nov 4. Cell Death Differ. 2017. PMID: 27813531 Free PMC article.
-
Unconventional apoptosis of polymorphonuclear neutrophils (PMN): staurosporine delays exposure of phosphatidylserine and prevents phagocytosis by MΦ-2 macrophages of PMN.Clin Exp Immunol. 2015 Jan;179(1):75-84. doi: 10.1111/cei.12412. Clin Exp Immunol. 2015. PMID: 24995908 Free PMC article.
-
BH3-only protein Bim is associated with the degree of Helicobacter pylori-induced gastritis and is localized to the mitochondria of inflammatory cells in the gastric mucosa.Int J Med Microbiol. 2015 Sep;305(6):553-62. doi: 10.1016/j.ijmm.2015.07.002. Epub 2015 Jul 9. Int J Med Microbiol. 2015. PMID: 26197709 Free PMC article.
References
-
- Metcalf D. The molecular control of granulocytes and macrophages. Ciba Found. Symp. 1997;204:40–50. - PubMed
-
- Mollinedo F., Borregaard N., Boxer L.A. Novel trends in neutrophil structure, function and development. Immunol. Today. 1999;20:535–537. - PubMed
-
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today. 1993;14:131–136. - PubMed
-
- Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. - PubMed
-
- Moulding D.A., Quayle J.A., Hart C.A., Edwards S.W. Mcl-1 expression in human neutrophilsregulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

