Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation
- PMID: 10958690
- PMCID: PMC88770
- DOI: 10.1128/MCB.20.18.6945-6957.2000
Deficiency of PTEN in Jurkat T cells causes constitutive localization of Itk to the plasma membrane and hyperresponsiveness to CD3 stimulation
Abstract
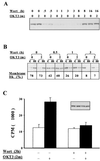
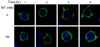
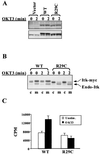
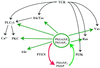
Pleckstrin homology (PH) domain binding to D3-phosphorylated phosphatidylinositides (PI) provides a reversible means of recruiting proteins to the plasma membrane, with the resultant change in subcellular localization playing a key role in the activation of multiple intracellular signaling pathways. Previously we found that the T-cell-specific PH domain-containing kinase Itk is constitutively membrane associated in Jurkat T cells. This distribution was unexpected given that the closely related B-cell kinase, Btk, is almost exclusively cytosolic. In addition to constitutive membrane association of Itk, unstimulated JTAg T cells also exhibited constitutive phosphorylation of Akt on Ser-473, an indication of elevated basal levels of the phosphatidylinositol 3-kinase (PI3K) products PI-3,4-P(2) and PI-3,4,5-P(3) in the plasma membrane. Here we describe a defect in expression of the D3 phosphoinositide phosphatase, PTEN, in Jurkat and JTAg T cells that leads to unregulated PH domain interactions with the plasma membrane. Inhibition of D3 phosphorylation by PI3K inhibitors, or by expression of PTEN, blocked constitutive phosphorylation of Akt on Ser-473 and caused Itk to redistribute to the cytosol. The PTEN-deficient cells were also hyperresponsive to T-cell receptor (TCR) stimulation, as measured by Itk kinase activity, tyrosine phosphorylation of phospholipase C-gamma1, and activation of Erk compared to those in PTEN-replete cells. These data support the idea that PH domain-mediated association with the plasma membrane is required for Itk activation, provide evidence for a negative regulatory role of PTEN in TCR stimulation, and suggest that signaling models based on results from Jurkat T-cell lines may underestimate the role of PI3K in TCR signaling.
Figures


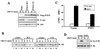


Similar articles
-
Evidence that SHIP-1 contributes to phosphatidylinositol 3,4,5-trisphosphate metabolism in T lymphocytes and can regulate novel phosphoinositide 3-kinase effectors.J Immunol. 2002 Nov 15;169(10):5441-50. doi: 10.4049/jimmunol.169.10.5441. J Immunol. 2002. PMID: 12421919
-
PTEN permits acute increases in D3-phosphoinositide levels following TCR stimulation but inhibits distal signaling events by reducing the basal activity of Akt.Eur J Immunol. 2004 Nov;34(11):3165-75. doi: 10.1002/eji.200425206. Eur J Immunol. 2004. PMID: 15468057
-
The tumor suppressor PTEN regulates T cell survival and antigen receptor signaling by acting as a phosphatidylinositol 3-phosphatase.J Immunol. 2000 Feb 15;164(4):1934-9. doi: 10.4049/jimmunol.164.4.1934. J Immunol. 2000. PMID: 10657643
-
Signaling pathways of D3-phosphoinositide-binding kinases in T cells and their regulation by PTEN.Semin Immunol. 2002 Feb;14(1):27-36. doi: 10.1006/smim.2001.0339. Semin Immunol. 2002. PMID: 11884228 Review.
-
Lipid phosphatases in the regulation of T cell activation: living up to their PTEN-tial.Immunol Rev. 2003 Apr;192:80-97. doi: 10.1034/j.1600-065x.2003.00013.x. Immunol Rev. 2003. PMID: 12670397 Review.
Cited by
-
The class I PI3K/Akt pathway is critical for cancer cell survival in dogs and offers an opportunity for therapeutic intervention.BMC Vet Res. 2012 May 30;8:73. doi: 10.1186/1746-6148-8-73. BMC Vet Res. 2012. PMID: 22647622 Free PMC article.
-
Akt-dependent phosphorylation specifically regulates Cot induction of NF-kappa B-dependent transcription.Mol Cell Biol. 2002 Aug;22(16):5962-74. doi: 10.1128/MCB.22.16.5962-5974.2002. Mol Cell Biol. 2002. PMID: 12138205 Free PMC article.
-
Lipid signaling in T-cell development and function.Cold Spring Harb Perspect Biol. 2010 Nov;2(11):a002428. doi: 10.1101/cshperspect.a002428. Epub 2010 Oct 13. Cold Spring Harb Perspect Biol. 2010. PMID: 20943760 Free PMC article. Review.
-
Phosphoinositide and inositol phosphate analysis in lymphocyte activation.Curr Protoc Immunol. 2009 Nov;Chapter 11:11.1.1-11.1.46. doi: 10.1002/0471142735.im1101s87. Curr Protoc Immunol. 2009. PMID: 19918943 Free PMC article.
-
TRAF3 in T Cells Restrains Negative Regulators of LAT to Promote TCR/CD28 Signaling.J Immunol. 2021 Jul 1;207(1):322-332. doi: 10.4049/jimmunol.2001220. Epub 2021 Jun 18. J Immunol. 2021. PMID: 34145060 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
