Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts
- PMID: 10953004
- PMCID: PMC2175273
- DOI: 10.1083/jcb.150.4.797
Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts
Abstract
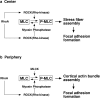
ROCK (Rho-kinase), an effector molecule of RhoA, phosphorylates the myosin binding subunit (MBS) of myosin phosphatase and inhibits the phosphatase activity. This inhibition increases phosphorylation of myosin light chain (MLC) of myosin II, which is suggested to induce RhoA-mediated assembly of stress fibers and focal adhesions. ROCK is also known to directly phosphorylate MLC in vitro; however, the physiological significance of this MLC kinase activity is unknown. It is also not clear whether MLC phosphorylation alone is sufficient for the assembly of stress fibers and focal adhesions. We have developed two reagents with opposing effects on myosin phosphatase. One is an antibody against MBS that is able to inhibit myosin phosphatase activity. The other is a truncation mutant of MBS that constitutively activates myosin phosphatase. Through microinjection of these two reagents followed by immunofluorescence with a specific antibody against phosphorylated MLC, we have found that MLC phosphorylation is both necessary and sufficient for the assembly of stress fibers and focal adhesions in 3T3 fibroblasts. The assembly of stress fibers in the center of cells requires ROCK activity in addition to the inhibition of myosin phosphatase, suggesting that ROCK not only inhibits myosin phosphatase but also phosphorylates MLC directly in the center of cells. At the cell periphery, on the other hand, MLCK but not ROCK appears to be the kinase responsible for phosphorylating MLC. These results suggest that ROCK and MLCK play distinct roles in spatial regulation of MLC phosphorylation.
Figures


Similar articles
-
Distinct roles of MLCK and ROCK in the regulation of membrane protrusions and focal adhesion dynamics during cell migration of fibroblasts.J Cell Biol. 2004 Feb 2;164(3):427-39. doi: 10.1083/jcb.200306172. J Cell Biol. 2004. PMID: 14757754 Free PMC article.
-
Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase).Science. 1996 Jul 12;273(5272):245-8. doi: 10.1126/science.273.5272.245. Science. 1996. PMID: 8662509
-
Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo.J Cell Biol. 1999 Nov 29;147(5):1023-38. doi: 10.1083/jcb.147.5.1023. J Cell Biol. 1999. PMID: 10579722 Free PMC article.
-
Role of myosin light chain phosphorylation in the regulation of cytokinesis.Cell Struct Funct. 2001 Dec;26(6):639-44. doi: 10.1247/csf.26.639. Cell Struct Funct. 2001. PMID: 11942620 Review.
-
Regulation of myosin II during cytokinesis in higher eukaryotes.Trends Cell Biol. 2005 Jul;15(7):371-7. doi: 10.1016/j.tcb.2005.05.004. Trends Cell Biol. 2005. PMID: 15935670 Review.
Cited by
-
Cell Response in Free-Packed Granular Systems.ACS Appl Mater Interfaces. 2022 Sep 14;14(36):40469-40480. doi: 10.1021/acsami.1c24095. Epub 2022 Aug 31. ACS Appl Mater Interfaces. 2022. PMID: 36044384 Free PMC article.
-
Loss of cortactin causes endothelial barrier dysfunction via disturbed adrenomedullin secretion and actomyosin contractility.Sci Rep. 2016 Jun 30;6:29003. doi: 10.1038/srep29003. Sci Rep. 2016. PMID: 27357373 Free PMC article.
-
Adhesion dynamics at a glance.J Cell Sci. 2011 Dec 1;124(Pt 23):3923-7. doi: 10.1242/jcs.095653. J Cell Sci. 2011. PMID: 22194302 Free PMC article. No abstract available.
-
Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia.Lab Invest. 2007 Aug;87(8):807-17. doi: 10.1038/labinvest.3700595. Epub 2007 Jun 18. Lab Invest. 2007. PMID: 17572689 Free PMC article.
-
Phosphorylation of focal adhesion kinase (FAK) on Ser732 is induced by rho-dependent kinase and is essential for proline-rich tyrosine kinase-2-mediated phosphorylation of FAK on Tyr407 in response to vascular endothelial growth factor.Mol Biol Cell. 2006 Aug;17(8):3508-20. doi: 10.1091/mbc.e05-12-1158. Epub 2006 Jun 7. Mol Biol Cell. 2006. PMID: 16760434 Free PMC article.
References
-
- Alessi D., MacDougall L.K., Sola M.M., Ikebe M., Cohen P. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur. J. Biochem. 1992;210:1023–1035. - PubMed
-
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., Matsuura Y., Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. - PubMed
-
- Amano M., Chihara K., Kimura K., Fukata Y., Nakamura N., Matsuura Y., Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Blatter D.P., Garner F., Van Slyke K., Bradley A. Quantitative electrophoresis in polyacrylamide gels of 2-40% J. Chromatogr. 1972;64:147–155.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

