Ca2+-dependent regulation of synaptic SNARE complex assembly via a calmodulin- and phospholipid-binding domain of synaptobrevin
- PMID: 10944231
- PMCID: PMC16927
- DOI: 10.1073/pnas.97.17.9695
Ca2+-dependent regulation of synaptic SNARE complex assembly via a calmodulin- and phospholipid-binding domain of synaptobrevin
Abstract
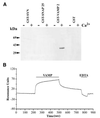
Synaptic core complex formation is an essential step in exocytosis, and assembly into a superhelical structure may drive synaptic vesicle fusion. To ascertain how Ca(2+) could regulate this process, we examined calmodulin binding to recombinant core complex components. Surface plasmon resonance and pull-down assays revealed Ca(2+)-dependent calmodulin binding (K(d) = 500 nM) to glutathione S-transferase fusion proteins containing synaptobrevin (VAMP 2) domains but not to syntaxin 1 or synaptosomal-associated protein of 25 kDa (SNAP-25). Deletion mutations, tetanus toxin cleavage, and peptide synthesis localized the calmodulin-binding domain to VAMP(77-94), immediately C-terminal to the tetanus toxin cleavage site (Q(76)-F(77)). In isolated synaptic vesicles, Ca(2+)/calmodulin protected native membrane-inserted VAMP from proteolysis by tetanus toxin. Assembly of a (35)S-SNAP-25, syntaxin 1 GST-VAMP(1-96) complex was inhibited by Ca(2+)/calmodulin, but assembly did not mask subsequent accessibility of the calmodulin-binding domain. The same domain contains a predicted phospholipid interaction site. SPR revealed calcium-independent interactions between VAMP(77-94) and liposomes containing phosphatidylserine, which blocked calmodulin binding. Circular dichroism spectroscopy demonstrated that the calmodulin/phospholipid-binding peptide displayed a significant increase in alphahelical content in a hydrophobic environment. These data provide insight into the mechanisms by which Ca(2+) may regulate synaptic core complex assembly and protein interactions with membrane bilayers during exocytosis.
Figures



Similar articles
-
Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion.Biol Cell. 2003 Oct;95(7):459-64. doi: 10.1016/s0248-4900(03)00076-5. Biol Cell. 2003. PMID: 14597264 Review.
-
Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis.EMBO J. 2002 Aug 1;21(15):3970-9. doi: 10.1093/emboj/cdf404. EMBO J. 2002. PMID: 12145198 Free PMC article.
-
Differential phosphorylation of syntaxin and synaptosome-associated protein of 25 kDa (SNAP-25) isoforms.J Neurochem. 1999 Feb;72(2):614-24. doi: 10.1046/j.1471-4159.1999.0720614.x. J Neurochem. 1999. PMID: 9930733
-
Effect of mutations in vesicle-associated membrane protein (VAMP) on the assembly of multimeric protein complexes.J Neurosci. 1997 Mar 1;17(5):1596-603. doi: 10.1523/JNEUROSCI.17-05-01596.1997. J Neurosci. 1997. PMID: 9030619 Free PMC article.
-
Interactions between presynaptic calcium channels and proteins implicated in synaptic vesicle trafficking and exocytosis.J Bioenerg Biomembr. 1998 Aug;30(4):347-56. doi: 10.1023/a:1021937605818. J Bioenerg Biomembr. 1998. PMID: 9758331 Review.
Cited by
-
Calmodulin regulates intracellular trafficking of epidermal growth factor receptor and the MAPK signaling pathway.Mol Biol Cell. 2002 Jun;13(6):2057-68. doi: 10.1091/mbc.01-12-0571. Mol Biol Cell. 2002. PMID: 12058069 Free PMC article.
-
Intracellular Ca2+ signaling and preimplantation development.Adv Exp Med Biol. 2015;843:151-71. doi: 10.1007/978-1-4939-2480-6_6. Adv Exp Med Biol. 2015. PMID: 25956298 Free PMC article. Review.
-
Productive hemifusion intermediates in fast vesicle fusion driven by neuronal SNAREs.Biophys J. 2008 Feb 15;94(4):1303-14. doi: 10.1529/biophysj.107.107896. Epub 2007 Oct 19. Biophys J. 2008. PMID: 17951297 Free PMC article.
-
Evidence that electrostatic interactions between vesicle-associated membrane protein 2 and acidic phospholipids may modulate the fusion of transport vesicles with the plasma membrane.Mol Biol Cell. 2009 Dec;20(23):4910-9. doi: 10.1091/mbc.e09-04-0284. Epub 2009 Oct 7. Mol Biol Cell. 2009. PMID: 19812247 Free PMC article.
-
Retrograde trafficking and quality control of yeast synaptobrevin, Snc1, are conferred by its transmembrane domain.Mol Biol Cell. 2019 Jul 1;30(14):1729-1742. doi: 10.1091/mbc.E19-02-0117. Epub 2019 May 8. Mol Biol Cell. 2019. PMID: 31067149 Free PMC article.
References
-
- Sollner T, Bennett M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. - PubMed
-
- Sollner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. - PubMed
-
- Schiavo G, Benfenati F, Poulain B, Rossetto O, Polverino de Laureto P, DasGupta B R, Montecucco C. Nature (London) 1992;359:832–835. - PubMed
-
- Niemann H, Blasi J, Jahn R. Trends Cell Biol. 1994;4:179–185. - PubMed
-
- Sutton R B, Fasshauer D, Jahn R, Brunger A T. Nature (London) 1998;395:347–353. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

