A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion
- PMID: 10944212
- PMCID: PMC16876
- DOI: 10.1073/pnas.97.17.9402
A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion
Abstract
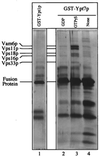
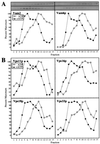
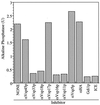
Yeast vacuoles undergo priming, docking, and homotypic fusion, although little has been known of the connections between these reactions. Vacuole-associated Vam2p and Vam6p (Vam2/6p) are components of a 65S complex containing SNARE proteins. Upon priming by Sec18p/NSF and ATP, Vam2/6p is released as a 38S subcomplex that binds Ypt7p to initiate docking. We now report that the 38S complex consists of both Vam2/6p and the class C Vps proteins [Reider, S. E. and Emr, S. D. (1997) Mol. Biol. Cell 8, 2307-2327]. This complex includes Vps33p, a member of the Sec1 family of proteins that bind t-SNAREs. We term this 38S complex HOPS, for homotypic fusion and vacuole protein sorting. This unexpected finding explains how Vam2/6p associates with SNAREs and provides a mechanistic link of the class C Vps proteins to Ypt/Rab action. HOPS initially associates with vacuole SNAREs in "cis" and, after release by priming, hops to Ypt7p, activating this Ypt/Rab switch to initiate docking.
Figures

Similar articles
-
The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein.J Cell Biol. 2000 Mar 20;148(6):1231-8. doi: 10.1083/jcb.148.6.1231. J Cell Biol. 2000. PMID: 10725336 Free PMC article.
-
New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion.J Cell Biol. 2000 Oct 30;151(3):551-62. doi: 10.1083/jcb.151.3.551. J Cell Biol. 2000. PMID: 11062257 Free PMC article.
-
A new role for a SNARE protein as a regulator of the Ypt7/Rab-dependent stage of docking.Proc Natl Acad Sci U S A. 2000 Aug 1;97(16):8889-91. doi: 10.1073/pnas.160269997. Proc Natl Acad Sci U S A. 2000. PMID: 10908678 Free PMC article.
-
Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles.Annu Rev Cell Dev Biol. 2010;26:115-36. doi: 10.1146/annurev-cellbio-100109-104131. Annu Rev Cell Dev Biol. 2010. PMID: 20521906 Review.
-
Involvement of LMA1 and GATE-16 family members in intracellular membrane dynamics.Biochim Biophys Acta. 2003 Aug 18;1641(2-3):145-56. doi: 10.1016/s0167-4889(03)00086-7. Biochim Biophys Acta. 2003. PMID: 12914955 Review.
Cited by
-
Yeast lipin 1 orthologue pah1p regulates vacuole homeostasis and membrane fusion.J Biol Chem. 2012 Jan 13;287(3):2221-36. doi: 10.1074/jbc.M111.317420. Epub 2011 Nov 25. J Biol Chem. 2012. PMID: 22121197 Free PMC article.
-
News and Views into the SNARE Complexity in Arabidopsis.Front Plant Sci. 2012 Feb 10;3:28. doi: 10.3389/fpls.2012.00028. eCollection 2012. Front Plant Sci. 2012. PMID: 23018380 Free PMC article.
-
Structural basis of Vps33A recruitment to the human HOPS complex by Vps16.Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13345-50. doi: 10.1073/pnas.1307074110. Epub 2013 Jul 30. Proc Natl Acad Sci U S A. 2013. PMID: 23901104 Free PMC article.
-
Chaperoning SNARE assembly and disassembly.Nat Rev Mol Cell Biol. 2016 Aug;17(8):465-79. doi: 10.1038/nrm.2016.65. Epub 2016 Jun 15. Nat Rev Mol Cell Biol. 2016. PMID: 27301672 Free PMC article. Review.
-
Endosomal maturation by Rab conversion in Aspergillus nidulans is coupled to dynein-mediated basipetal movement.Mol Biol Cell. 2012 May;23(10):1889-901. doi: 10.1091/mbc.E11-11-0925. Epub 2012 Mar 28. Mol Biol Cell. 2012. PMID: 22456509 Free PMC article.
References
-
- Rothman J H, Stevens T. Cell. 1986;47:1041–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

