A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition
- PMID: 10938129
- PMCID: PMC86127
- DOI: 10.1128/MCB.20.17.6537-6549.2000
A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition
Abstract
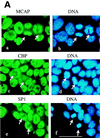
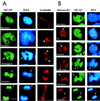
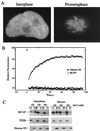
We describe a novel nuclear factor called mitotic chromosome-associated protein (MCAP), which belongs to the poorly understood BET subgroup of the bromodomain superfamily. Expression of the 200-kDa MCAP was linked to cell division, as it was induced by growth stimulation and repressed by growth inhibition. The most notable feature of MCAP was its association with chromosomes during mitosis, observed at a time when the majority of nuclear regulatory factors were released into the cytoplasm, coinciding with global cessation of transcription. Indicative of its predominant interaction with euchromatin, MCAP localized on mitotic chromosomes with exquisite specificity: (i) MCAP-chromosome association became evident subsequent to the initiation of histone H3 phosphorylation and early chromosomal condensation; and (ii) MCAP was absent from centromeres, the sites of heterochromatin. Supporting a role for MCAP in G(2)/M transition, microinjection of anti-MCAP antibody into HeLa cell nuclei completely inhibited the entry into mitosis, without abrogating the ongoing DNA replication. These results suggest that MCAP plays a role in a process governing chromosomal dynamics during mitosis.
Figures








Similar articles
-
A human condensin complex containing hCAP-C-hCAP-E and CNAP1, a homolog of Xenopus XCAP-D2, colocalizes with phosphorylated histone H3 during the early stage of mitotic chromosome condensation.Mol Cell Biol. 2000 Sep;20(18):6996-7006. doi: 10.1128/MCB.20.18.6996-7006.2000. Mol Cell Biol. 2000. PMID: 10958694 Free PMC article.
-
Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation.J Cell Sci. 1998 Dec;111 ( Pt 23):3497-506. doi: 10.1242/jcs.111.23.3497. J Cell Sci. 1998. PMID: 9811564
-
Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription.Mol Biol Cell. 2009 Dec;20(23):4899-909. doi: 10.1091/mbc.e09-05-0380. Epub 2009 Oct 7. Mol Biol Cell. 2009. PMID: 19812244 Free PMC article.
-
Differential regulation of maternal and paternal chromosome condensation in mitotic zygotes.J Cell Sci. 2002 Jul 15;115(Pt 14):2931-40. doi: 10.1242/jcs.115.14.2931. J Cell Sci. 2002. PMID: 12082153
-
Mitotic Chromosome Mechanics: How Cells Segregate Their Genome.Trends Cell Biol. 2019 Sep;29(9):717-726. doi: 10.1016/j.tcb.2019.05.007. Epub 2019 Jun 20. Trends Cell Biol. 2019. PMID: 31230958 Review.
Cited by
-
MacroH2A1.2 deficiency leads to neural stem cell differentiation defects and autism-like behaviors.EMBO Rep. 2021 Jul 5;22(7):e52150. doi: 10.15252/embr.202052150. Epub 2021 May 27. EMBO Rep. 2021. PMID: 34046991 Free PMC article.
-
Bromodomain and Extraterminal Protein Inhibition Blocks Growth of Triple-negative Breast Cancers through the Suppression of Aurora Kinases.J Biol Chem. 2016 Nov 4;291(45):23756-23768. doi: 10.1074/jbc.M116.738666. Epub 2016 Sep 20. J Biol Chem. 2016. PMID: 27650498 Free PMC article.
-
NUT rearrangement is uncommon in human thymic epithelial tumors.J Thorac Oncol. 2012 Apr;7(4):744-50. doi: 10.1097/JTO.0b013e3182460f8f. J Thorac Oncol. 2012. PMID: 22425924 Free PMC article.
-
A modular CRISPR screen identifies individual and combination pathways contributing to HIV-1 latency.PLoS Pathog. 2023 Jan 27;19(1):e1011101. doi: 10.1371/journal.ppat.1011101. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36706161 Free PMC article.
-
BRD4 short isoform interacts with RRP1B, SIPA1 and components of the LINC complex at the inner face of the nuclear membrane.PLoS One. 2013 Nov 19;8(11):e80746. doi: 10.1371/journal.pone.0080746. eCollection 2013. PLoS One. 2013. PMID: 24260471 Free PMC article.
References
-
- Beck S, Hanson I, Kelly A, Pappin D J, Trowsdale J. A homologue of the Drosophila female sterile homeotic (fsh) gene in the class II region of the human MHC. DNA Seq. 1992;2:203–210. - PubMed
-
- Coligan J, Kruisbeek A, Shevach E, Margulies D, editors. Current protocols in immunology. New York, N.Y: John Wiley & Sons; 1997.
-
- Coverley D, Laskey R A. Regulation of eukaryotic DNA replication. Annu Rev Biochem. 1994;63:745–776. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
