A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo
- PMID: 10934224
- PMCID: PMC2193213
- DOI: 10.1084/jem.192.3.359
A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo
Abstract
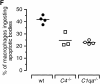
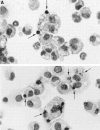
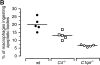
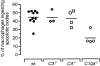
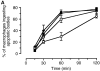
The strongest susceptibility genes for the development of systemic lupus erythematosus (SLE) in humans are null mutants of classical pathway complement proteins. There is a hierarchy of disease susceptibility and severity according to the position of the missing protein in the activation pathway, with the severest disease associated with C1q deficiency. Here we demonstrate, using novel in vivo models of apoptotic cell clearance during sterile peritonitis, a similar hierarchical role for classical pathway complement proteins in vivo in the clearance of apoptotic cells by macrophages. Our results constitute the first demonstration of an impairment in the phagocytosis of apoptotic cells by macrophages in vivo in a mammalian system. Apoptotic cells are thought to be a major source of the autoantigens of SLE, and impairment of their removal by complement may explain the link between hereditary complement deficiency and the development of SLE.
Figures
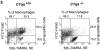


Similar articles
-
IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo.Autoimmunity. 2005 Jun;38(4):259-64. doi: 10.1080/08916930500124452. Autoimmunity. 2005. PMID: 16206508
-
Complement classical pathway components are all important in clearance of apoptotic and secondary necrotic cells.Clin Exp Immunol. 2009 May;156(2):303-11. doi: 10.1111/j.1365-2249.2009.03896.x. Epub 2009 Mar 2. Clin Exp Immunol. 2009. PMID: 19302245 Free PMC article.
-
C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells.J Immunol. 2009 Nov 15;183(10):6175-85. doi: 10.4049/jimmunol.0902232. Epub 2009 Oct 28. J Immunol. 2009. PMID: 19864605 Free PMC article.
-
C1q, autoimmunity and apoptosis.Immunobiology. 2002 Sep;205(4-5):395-406. doi: 10.1078/0171-2985-00141. Immunobiology. 2002. PMID: 12396002 Review.
-
C1q knock-out mice for the study of complement deficiency in autoimmune disease.Exp Clin Immunogenet. 1998;15(4):231-4. doi: 10.1159/000019076. Exp Clin Immunogenet. 1998. PMID: 10072632 Review.
Cited by
-
670-nm light treatment reduces complement propagation following retinal degeneration.J Neuroinflammation. 2012 Nov 26;9:257. doi: 10.1186/1742-2094-9-257. J Neuroinflammation. 2012. PMID: 23181358 Free PMC article.
-
Complement in Lupus Nephritis: New Perspectives.Kidney Dis (Basel). 2015 Sep;1(2):91-9. doi: 10.1159/000431278. Epub 2015 Jun 30. Kidney Dis (Basel). 2015. PMID: 27536669 Free PMC article. Review.
-
Mechanisms of Photoreceptor Death in Retinitis Pigmentosa.Genes (Basel). 2020 Sep 24;11(10):1120. doi: 10.3390/genes11101120. Genes (Basel). 2020. PMID: 32987769 Free PMC article. Review.
-
Kidney Involvement in Hypocomplementemic Urticarial Vasculitis Syndrome-A Case-Based Review.J Clin Med. 2020 Jul 6;9(7):2131. doi: 10.3390/jcm9072131. J Clin Med. 2020. PMID: 32640739 Free PMC article. Review.
-
Deoxyribonuclease 1-Mediated Clearance of Circulating Chromatin Prevents From Immune Cell Activation and Pro-inflammatory Cytokine Production, a Phenomenon Amplified by Low Trap1 Activity: Consequences for Systemic Lupus Erythematosus.Front Immunol. 2021 Mar 4;12:613597. doi: 10.3389/fimmu.2021.613597. eCollection 2021. Front Immunol. 2021. PMID: 33746957 Free PMC article.
References
-
- Morgan B.P., Walport M.J. Complement deficiency and disease. Immunol. Today. 1991;12:301–306. - PubMed
-
- Botto M., Dell'Agnola C., Bygrave A.E., Thompson E.M., Cook H.T., Petry F., Loos M., Pandolfi P.P., Walport M.J. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 1998;19:56–59. - PubMed
-
- Korb L.C., Ahearn J.M. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytescomplement deficiency and systemic lupus erythematosus revisited. J. Immunol. 1997;158:4525–4528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

