Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity
- PMID: 10933678
- PMCID: PMC112301
- DOI: 10.1128/jvi.74.17.7730-7737.2000
Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity
Abstract
The interaction between the viral protein linked to the genome (VPg) of turnip mosaic potyvirus (TuMV) and the translation eukaryotic initiation factor eIF(iso)4E of Arabidopsis thaliana has previously been reported. eIF(iso)4E binds the cap structure (m(7)GpppN, where N is any nucleotide) of mRNAs and has an important role in the regulation in the initiation of translation. In the present study, it was shown that not only did VPg bind eIF(iso)4E but it also interacted with the eIF4E isomer of A. thaliana as well as with eIF(iso)4E of Triticum aestivum (wheat). The interaction domain on VPg was mapped to a stretch of 35 amino acids, and substitution of an aspartic acid residue found within this region completely abolished the interaction. The cap analogue m(7)GTP, but not GTP, inhibited VPg-eIF(iso)4E complex formation, suggesting that VPg and cellular mRNAs compete for eIF(iso)4E binding. The biological significance of this interaction was investigated. Brassica perviridis plants were infected with a TuMV infectious cDNA (p35Tunos) and p35TuD77N, a mutant which contained the aspartic acid substitution in the VPg domain that abolished the interaction with eIF(iso)4E. After 20 days, plants bombarded with p35Tunos showed viral symptoms, while plants bombarded with p35TuD77N remained symptomless. These results suggest that VPg-eIF(iso)4E interaction is a critical element for virus production.
Figures

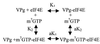

Similar articles
-
Variability in eukaryotic initiation factor iso4E in Brassica rapa influences interactions with the viral protein linked to the genome of Turnip mosaic virus.Sci Rep. 2018 Sep 11;8(1):13588. doi: 10.1038/s41598-018-31739-1. Sci Rep. 2018. PMID: 30206242 Free PMC article.
-
Potyvirus terminal protein VPg, effector of host eukaryotic initiation factor eIF4E.Biochimie. 2006 Jul;88(7):887-96. doi: 10.1016/j.biochi.2006.02.012. Epub 2006 Mar 31. Biochimie. 2006. PMID: 16626853
-
Transgenic Brassica rapa plants over-expressing eIF(iso)4E variants show broad-spectrum Turnip mosaic virus (TuMV) resistance.Mol Plant Pathol. 2014 Aug;15(6):615-26. doi: 10.1111/mpp.12120. Epub 2014 Apr 15. Mol Plant Pathol. 2014. PMID: 24417952 Free PMC article.
-
Cap binding complexes and cellular growth control.Biochimie. 1995;77(1-2):40-4. doi: 10.1016/0300-9084(96)88102-8. Biochimie. 1995. PMID: 7599274 Review.
-
[Gene expression of human eukaryotic initiation factor-4E for protein synthesis and study of its recognition mechanism of mRNA cap structure].Yakugaku Zasshi. 1995 Jun;115(6):401-19. doi: 10.1248/yakushi1947.115.6_401. Yakugaku Zasshi. 1995. PMID: 7666354 Review. Japanese.
Cited by
-
An induced mutation in tomato eIF4E leads to immunity to two potyviruses.PLoS One. 2010 Jun 25;5(6):e11313. doi: 10.1371/journal.pone.0011313. PLoS One. 2010. PMID: 20593023 Free PMC article.
-
Relationship between Sugarcane eIF4E Gene and Resistance against Sugarcane Streak Mosaic Virus.Plants (Basel). 2023 Jul 28;12(15):2805. doi: 10.3390/plants12152805. Plants (Basel). 2023. PMID: 37570959 Free PMC article.
-
Sapovirus translation requires an interaction between VPg and the cap binding protein eIF4E.J Virol. 2014 Nov;88(21):12213-21. doi: 10.1128/JVI.01650-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142584 Free PMC article.
-
Biotechnological strategies and tools for Plum pox virus resistance: trans-, intra-, cis-genesis, and beyond.Front Plant Sci. 2015 Jun 8;6:379. doi: 10.3389/fpls.2015.00379. eCollection 2015. Front Plant Sci. 2015. PMID: 26106397 Free PMC article. Review.
-
Elucidation of virus-host interactions to enhance resistance breeding for control of virus diseases in potato.Breed Sci. 2015 Mar;65(1):69-76. doi: 10.1270/jsbbs.65.69. Epub 2015 Mar 1. Breed Sci. 2015. PMID: 25931981 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

