Syntaxin 17 is abundant in steroidogenic cells and implicated in smooth endoplasmic reticulum membrane dynamics
- PMID: 10930465
- PMCID: PMC14951
- DOI: 10.1091/mbc.11.8.2719
Syntaxin 17 is abundant in steroidogenic cells and implicated in smooth endoplasmic reticulum membrane dynamics
Abstract
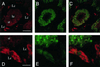
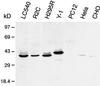
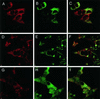
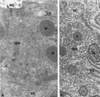
The endoplasmic reticulum (ER) consists of subcompartments that have distinct protein constituents, morphological appearances, and functions. To understand the mechanisms that regulate the intricate and dynamic organization of the endoplasmic reticulum, it is important to identify and characterize the molecular machinery involved in the assembly and maintenance of the different subcompartments. Here we report that syntaxin 17 is abundantly expressed in steroidogenic cell types and specifically localizes to smooth membranes of the ER. By immunoprecipitation analyses, syntaxin 17 exists in complexes with a syntaxin regulatory protein, rsly1, and/or two intermediate compartment SNARE proteins, rsec22b and rbet1. Furthermore, we found that syntaxin 17 is anchored to the smooth endoplasmic reticulum through an unusual mechanism, requiring two adjacent hydrophobic domains near its carboxyl terminus. Converging lines of evidence indicate that syntaxin 17 functions in a vesicle-trafficking step to the smooth-surfaced tubular ER membranes that are abundant in steroidogenic cells.
Figures






Similar articles
-
Localization, dynamics, and protein interactions reveal distinct roles for ER and Golgi SNAREs.J Cell Biol. 1998 Jun 29;141(7):1489-502. doi: 10.1083/jcb.141.7.1489. J Cell Biol. 1998. PMID: 9647643 Free PMC article.
-
rsly1 binding to syntaxin 5 is required for endoplasmic reticulum-to-Golgi transport but does not promote SNARE motif accessibility.Mol Biol Cell. 2004 Jan;15(1):162-75. doi: 10.1091/mbc.e03-07-0535. Epub 2003 Oct 17. Mol Biol Cell. 2004. PMID: 14565970 Free PMC article.
-
Role of syntaxin 18 in the organization of endoplasmic reticulum subdomains.J Cell Sci. 2009 May 15;122(Pt 10):1680-90. doi: 10.1242/jcs.036103. Epub 2009 Apr 28. J Cell Sci. 2009. PMID: 19401338
-
SNAREs and membrane fusion in the Golgi apparatus.Biochim Biophys Acta. 1998 Aug 14;1404(1-2):9-31. doi: 10.1016/s0167-4889(98)00044-5. Biochim Biophys Acta. 1998. PMID: 9714710 Review.
-
Syntaxin 6: the promiscuous behaviour of a SNARE protein.Traffic. 2001 Sep;2(9):606-11. doi: 10.1034/j.1600-0854.2001.20903.x. Traffic. 2001. PMID: 11555414 Review.
Cited by
-
The Neuroproteomic Basis of Enhanced Perception and Processing of Brood Signals That Trigger Increased Reproductive Investment in Honeybee (Apis mellifera) Workers.Mol Cell Proteomics. 2020 Oct;19(10):1632-1648. doi: 10.1074/mcp.RA120.002123. Epub 2020 Jul 15. Mol Cell Proteomics. 2020. PMID: 32669299 Free PMC article.
-
ER-to-lysosome-associated degradation of proteasome-resistant ATZ polymers occurs via receptor-mediated vesicular transport.EMBO J. 2018 Sep 3;37(17):e99259. doi: 10.15252/embj.201899259. Epub 2018 Aug 3. EMBO J. 2018. PMID: 30076131 Free PMC article.
-
Legionella effector Lpg1137 shuts down ER-mitochondria communication through cleavage of syntaxin 17.Nat Commun. 2017 May 15;8:15406. doi: 10.1038/ncomms15406. Nat Commun. 2017. PMID: 28504273 Free PMC article.
-
Interfering with Autophagy: The Opposing Strategies Deployed by Legionella pneumophila and Coxiella burnetii Effector Proteins.Front Cell Infect Microbiol. 2020 Nov 5;10:599762. doi: 10.3389/fcimb.2020.599762. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33251162 Free PMC article. Review.
-
Mechanisms for exporting large-sized cargoes from the endoplasmic reticulum.Cell Mol Life Sci. 2015 Oct;72(19):3709-20. doi: 10.1007/s00018-015-1952-9. Epub 2015 Jun 17. Cell Mol Life Sci. 2015. PMID: 26082182 Free PMC article. Review.
References
-
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. - PubMed
-
- Brands R, Slot JW, Geuze HJ. Albumin localization in rat liver parenchymal cells. Eur J Cell Biol. 1983;32:99–107. - PubMed
-
- Dascher C, Balch WE. Mammalian Sly1 regulates syntaxin 5 function in endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:15866–15869. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

