Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta
- PMID: 10930463
- PMCID: PMC14949
- DOI: 10.1091/mbc.11.8.2691
Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta
Abstract
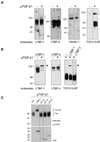
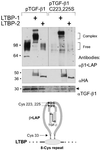
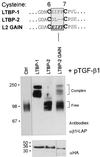
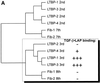
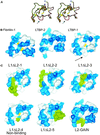
Transforming growth factor (TGF)-betas are secreted in large latent complexes consisting of TGF-beta, its N-terminal latency-associated peptide (LAP) propeptide, and latent TGF-beta binding protein (LTBP). LTBPs are required for secretion and subsequent deposition of TGF-beta into the extracellular matrix. TGF-beta1 associates with the 3(rd) 8-Cys repeat of LTBP-1 by LAP. All LTBPs, as well as fibrillins, contain multiple 8-Cys repeats. We analyzed the abilities of fibrillins and LTBPs to bind latent TGF-beta by their 8-Cys repeats. 8-Cys repeat was found to interact with TGF-beta1*LAP by direct cysteine bridging. LTBP-1 and LTBP-3 bound efficiently all TGF-beta isoforms, LTBP-4 had a much weaker binding capacity, whereas LTBP-2 as well as fibrillins -1 and -2 were negative. A short, specific TGF-beta binding motif was identified in the TGF-beta binding 8-Cys repeats. Deletion of this motif in the 3(rd) 8-Cys repeat of LTBP-1 resulted in loss of TGF-beta*LAP binding ability, while its inclusion in non-TGF-beta binding 3(rd) 8-Cys repeat of LTBP-2 resulted in TGF-beta binding. Molecular modeling of the 8-Cys repeats revealed a hydrophobic interaction surface and lack of three stabilizing hydrogen bonds introduced by the TGF-beta binding motif necessary for the formation of the TGF-beta*LAP - 8-Cys repeat complex inside the cells.
Figures

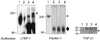
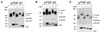


Similar articles
-
Latent transforming growth factor-beta binding proteins (LTBPs)--structural extracellular matrix proteins for targeting TGF-beta action.Cytokine Growth Factor Rev. 1999 Jun;10(2):99-117. doi: 10.1016/s1359-6101(99)00010-6. Cytokine Growth Factor Rev. 1999. PMID: 10743502 Review.
-
Association of the small latent transforming growth factor-beta with an eight cysteine repeat of its binding protein LTBP-1.EMBO J. 1996 Jan 15;15(2):245-53. EMBO J. 1996. PMID: 8617200 Free PMC article.
-
Amino acid requirements for formation of the TGF-beta-latent TGF-beta binding protein complexes.J Mol Biol. 2005 Jan 7;345(1):175-86. doi: 10.1016/j.jmb.2004.10.039. J Mol Biol. 2005. PMID: 15567420
-
Identification and characterization of a new latent transforming growth factor-beta-binding protein, LTBP-4.J Biol Chem. 1998 Jul 17;273(29):18459-69. doi: 10.1074/jbc.273.29.18459. J Biol Chem. 1998. PMID: 9660815
-
Latent TGF-β-binding proteins.Matrix Biol. 2015 Sep;47:44-53. doi: 10.1016/j.matbio.2015.05.005. Epub 2015 May 8. Matrix Biol. 2015. PMID: 25960419 Free PMC article. Review.
Cited by
-
Accuracy of novel diagnostic biomarkers for hepatocellular carcinoma: An update for clinicians (Review).Oncol Rep. 2016 Aug;36(2):613-25. doi: 10.3892/or.2016.4842. Epub 2016 Jun 1. Oncol Rep. 2016. PMID: 27278244 Free PMC article. Review.
-
Reevaluation of Pluripotent Cytokine TGF-β3 in Immunity.Int J Mol Sci. 2018 Aug 1;19(8):2261. doi: 10.3390/ijms19082261. Int J Mol Sci. 2018. PMID: 30071700 Free PMC article. Review.
-
Determination of the molecular basis of Marfan syndrome: a growth industry.J Clin Invest. 2004 Jul;114(2):161-3. doi: 10.1172/JCI22399. J Clin Invest. 2004. PMID: 15254580 Free PMC article.
-
Sequestration of latent TGF-β binding protein 1 into CADASIL-related Notch3-ECD deposits.Acta Neuropathol Commun. 2014 Aug 13;2:96. doi: 10.1186/s40478-014-0096-8. Acta Neuropathol Commun. 2014. PMID: 25190493 Free PMC article.
-
Fibrillin-containing microfibrils are key signal relay stations for cell function.J Cell Commun Signal. 2015 Dec;9(4):309-25. doi: 10.1007/s12079-015-0307-5. Epub 2015 Oct 8. J Cell Commun Signal. 2015. PMID: 26449569 Free PMC article.
References
-
- Bashir MM, Han MD, Abrams WR, Tucker T, Ma RI, Gibson M, Ritty T, Mecham R, Rosenbloom J. Analysis of the human gene encoding latent transforming growth factor-β-binding protein-2. Int J Biochem Cell Biol. 1996;28:531–542. - PubMed
-
- Dallas SL, Park-Snyder S, Miyazono K, Twardzik D, Mundy GR, Bonewald LF. Characterization and autoregulation of latent transforming growth factor-β (TGF-β) complexes in osteoblast-like cell lines: production of a latent complex lacking the latent TGF-β-binding protein. J Biol Chem. 1994;269:6815–6821. - PubMed
-
- D'Arrigo C, Burl S, Withers AP, Dobson H, Black C, Boxer M. TGF-β1 binding protein-like modules of fibrillin-1 and -2 mediate integrin-dependent cell adhesion. Connect Tissue Res. 1998;37:29–51. - PubMed
-
- Davis CG. The many faces of epidermal growth factor repeats. New Biol. 1990;2:410–419. - PubMed
-
- Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor-β 1 precursor by human furin convertase. J Biol Chem. 1995;270:10618–10624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

