Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons
- PMID: 10900020
- PMCID: PMC26999
- DOI: 10.1073/pnas.97.15.8629
Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons
Abstract
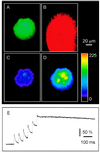
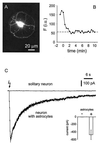
Astrocytes can release glutamate in a calcium-dependent manner and consequently signal to adjacent neurons. Whether this glutamate release pathway is used during physiological signaling or is recruited only under pathophysiological conditions is not well defined. One reason for this lack of understanding is the limited knowledge about the levels of calcium necessary to stimulate glutamate release from astrocytes and about how they compare with the range of physiological calcium levels in these cells. We used flash photolysis to raise internal calcium in astrocytes, while monitoring astrocytic calcium levels and glutamate, which evoked slow inward currents that were recorded electrophysiologically from single neurons grown on microislands of astrocytes. With this approach, we demonstrate that modest changes of astrocytic calcium, from 84 to 140 nM, evoke substantial glutamatergic currents in neighboring neurons (-391 pA), with a Hill coefficient of 2.1 to 2.7. Because the agonists glutamate, norepinephrine, and dopamine all raise calcium in astrocytes to levels exceeding 1.8 microM, these quantitative studies demonstrate that the astrocytic glutamate release pathway is engaged at physiological levels of internal calcium. Consequently, the calcium-dependent release of glutamate from astrocytes functions within an appropriate range of astrocytic calcium levels to be used as a signaling pathway within the functional nervous system.
Figures

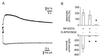

Comment in
-
Neural circuits in the 21st century: synaptic networks of neurons and glia.Proc Natl Acad Sci U S A. 2000 Jul 18;97(15):8196-7. doi: 10.1073/pnas.97.15.8196. Proc Natl Acad Sci U S A. 2000. PMID: 10899989 Free PMC article. No abstract available.
Similar articles
-
Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons.J Neurophysiol. 2005 Dec;94(6):4121-30. doi: 10.1152/jn.00448.2005. Epub 2005 Sep 14. J Neurophysiol. 2005. PMID: 16162834
-
Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ.Glia. 1999 Mar;26(1):1-11. doi: 10.1002/(sici)1098-1136(199903)26:1<1::aid-glia1>3.0.co;2-z. Glia. 1999. PMID: 10088667
-
Prostaglandin E(2) stimulates glutamate receptor-dependent astrocyte neuromodulation in cultured hippocampal cells.J Neurobiol. 1999 Nov 5;41(2):221-9. J Neurobiol. 1999. PMID: 10512979
-
Bidirectional astrocyte-neuron communication: the many roles of glutamate and ATP.Novartis Found Symp. 2006;276:208-17; discussion 217-21, 233-7, 275-81. doi: 10.1002/9780470032244.ch16. Novartis Found Symp. 2006. PMID: 16805432 Review.
-
Glutamate release from astrocytes as a non-synaptic mechanism for neuronal synchronization in the hippocampus.J Physiol Paris. 2006 Mar-May;99(2-3):98-102. doi: 10.1016/j.jphysparis.2005.12.008. J Physiol Paris. 2006. PMID: 16646155 Review.
Cited by
-
Enhanced astrocytic Ca2+ signals contribute to neuronal excitotoxicity after status epilepticus.J Neurosci. 2007 Oct 3;27(40):10674-84. doi: 10.1523/JNEUROSCI.2001-07.2007. J Neurosci. 2007. PMID: 17913901 Free PMC article.
-
Evidence for the existence of secretory granule (dense-core vesicle)-based inositol 1,4,5-trisphosphate-dependent Ca2+ signaling system in astrocytes.PLoS One. 2010 Aug 5;5(8):e11973. doi: 10.1371/journal.pone.0011973. PLoS One. 2010. PMID: 20700485 Free PMC article.
-
Mechanisms of glutamate release from astrocytes.Neurochem Int. 2008 Jan;52(1-2):142-54. doi: 10.1016/j.neuint.2007.06.005. Epub 2007 Jun 26. Neurochem Int. 2008. PMID: 17669556 Free PMC article. Review.
-
Flavonoids and astrocytes crosstalking: implications for brain development and pathology.Neurochem Res. 2010 Jul;35(7):955-66. doi: 10.1007/s11064-010-0144-0. Epub 2010 Mar 9. Neurochem Res. 2010. PMID: 20213345 Review.
-
Glutamate (mGluR-5) gene expression in brain regions of streptozotocin induced diabetic rats as a function of age: role in regulation of calcium release from the pancreatic islets in vitro.J Biomed Sci. 2009 Nov 10;16(1):99. doi: 10.1186/1423-0127-16-99. J Biomed Sci. 2009. PMID: 19903331 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

