Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells
- PMID: 10899112
- PMCID: PMC313987
- DOI: 10.1093/emboj/19.14.3576
Measles virus matrix protein specifies apical virus release and glycoprotein sorting in epithelial cells
Abstract
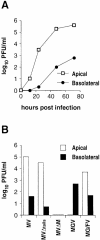
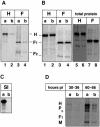
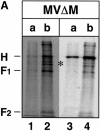
In polarized epithelial cells measles virus (MV) is predominantly released at the apical cell surface, irrespective of the sorting of its two envelope glycoproteins F and H. It has been reported previously that the viral matrix (M) protein modulates the fusogenic capacity of the viral envelope glycoproteins. Here, extant MV mutants and chimeras were used to determine the role of M protein in the transport of viral glycoproteins and release of progeny virions in polarized epithelial CaCo2 cells. In the absence of M, envelope glycoproteins are sorted to the basolateral surface, suggesting that they possess intrinsic basolateral sorting signals. However, interactions of M with the glycoprotein cytoplasmic tails allow M-glycoprotein co-segregation to the apical surface, suggesting a vectorial function of M to retarget the glycoproteins for apical virion release. Whereas this may allow virus airway shedding, the intrinsic sorting of the glycoproteins to the basolateral surface may account for systemic host infection by allowing efficient cell-cell fusion.
Figures








Similar articles
-
Entry and release of measles virus are polarized in epithelial cells.Virology. 1995 Jun 20;210(1):91-9. doi: 10.1006/viro.1995.1320. Virology. 1995. PMID: 7793085
-
Recombinant vesicular stomatitis virus expressing respiratory syncytial virus (RSV) glycoproteins: RSV fusion protein can mediate infection and cell fusion.Virology. 1999 Feb 1;254(1):81-91. doi: 10.1006/viro.1998.9535. Virology. 1999. PMID: 9927576
-
Basolateral sorting of human poliovirus receptor alpha involves an interaction with the mu1B subunit of the clathrin adaptor complex in polarized epithelial cells.Biochem Biophys Res Commun. 2001 Oct 5;287(4):941-8. doi: 10.1006/bbrc.2001.5660. Biochem Biophys Res Commun. 2001. PMID: 11573956
-
Cytoplasmic tails of bunyavirus Gn glycoproteins-Could they act as matrix protein surrogates?Virology. 2013 Mar 15;437(2):73-80. doi: 10.1016/j.virol.2013.01.001. Epub 2013 Jan 26. Virology. 2013. PMID: 23357734 Review.
-
Role of N- and O-glycans in polarized biosynthetic sorting.Am J Physiol Cell Physiol. 2006 Jan;290(1):C1-C10. doi: 10.1152/ajpcell.00333.2005. Am J Physiol Cell Physiol. 2006. PMID: 16338974 Review.
Cited by
-
Measles virus spreads in rat hippocampal neurons by cell-to-cell contact and in a polarized fashion.J Virol. 2002 Jun;76(11):5720-8. doi: 10.1128/jvi.76.11.5720-5728.2002. J Virol. 2002. PMID: 11992000 Free PMC article.
-
A polarized cell model for Chikungunya virus infection: entry and egress of virus occurs at the apical domain of polarized cells.PLoS Negl Trop Dis. 2014 Feb 20;8(2):e2661. doi: 10.1371/journal.pntd.0002661. eCollection 2014 Feb. PLoS Negl Trop Dis. 2014. PMID: 24587455 Free PMC article.
-
Paramyxovirus glycoprotein incorporation, assembly and budding: a three way dance for infectious particle production.Viruses. 2014 Aug 7;6(8):3019-54. doi: 10.3390/v6083019. Viruses. 2014. PMID: 25105277 Free PMC article. Review.
-
Co-assembly of viral envelope glycoproteins regulates their polarized sorting in neurons.PLoS Pathog. 2014 May 15;10(5):e1004107. doi: 10.1371/journal.ppat.1004107. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24831812 Free PMC article.
-
RNA interference with measles virus N, P, and L mRNAs efficiently prevents and with matrix protein mRNA enhances viral transcription.J Virol. 2006 Jun;80(12):5951-7. doi: 10.1128/JVI.02453-05. J Virol. 2006. PMID: 16731933 Free PMC article.
References
-
- Alfalah M., Jacob,R., Preuss,U., Zimmer,K.P., Naim,H. and Naim,H.Y. (1999) O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr. Biol., 9, 593–596. - PubMed
-
- Billeter M.A., Cattaneo,R., Spielhofer,P., Kaelin,K., Huber,M., Schmid,A., Baczko,K. and ter Meulen,V. (1994) Generation and properties of measles virus mutations typically associated with subacute sclerosing panencephalitis. Ann. N Y Acad. Sci., 724, 367–377. - PubMed
-
- Blau D.M. and Compans,R.W. (1995) Entry and release of measles virus are polarized in epithelial cells. Virology, 210, 91–99. - PubMed
-
- Bohn W., Rutter,G., Hohenberg,H., Mannweiler,K. and Nobis,P. (1986) Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology, 149, 91–106. - PubMed
-
- Brown D.A. and Rose,J.K. (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell, 68, 533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

