Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane
- PMID: 10893267
- PMCID: PMC2185562
- DOI: 10.1083/jcb.150.1.193
Morphogenic effects of ezrin require a phosphorylation-induced transition from oligomers to monomers at the plasma membrane
Abstract
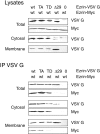
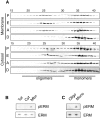
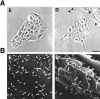
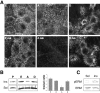
ERM (ezrin, radixin, moesin) proteins act as linkers between the plasma membrane and the actin cytoskeleton. An interaction between their NH(2)- and COOH-terminal domains occurs intramolecularly in closed monomers and intermolecularly in head-to-tail oligomers. In vitro, phosphorylation of a conserved threonine residue (T567 in ezrin) in the COOH-terminal domain of ERM proteins disrupts this interaction. Here, we have analyzed the role of this phosphorylation event in vivo, by deriving stable clones producing wild-type, T567A, and T567D ezrin from LLC-PK1 epithelial cells. We found that T567A ezrin was poorly associated with the cytoskeleton, but was able to form oligomers. In contrast, T567D ezrin was associated with the cytoskeleton, but its distribution was shifted from oligomers to monomers at the membrane. Moreover, production of T567D ezrin induced the formation of lamellipodia, membrane ruffles, and tufts of microvilli. Both T567A and T567D ezrin affected the development of multicellular epithelial structures. Collectively, these results suggest that phosphorylation of ERM proteins on this conserved threonine regulates the transition from membrane-bound oligomers to active monomers, which induce and are part of actin-rich membrane projections.
Figures



Similar articles
-
Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation.J Cell Biol. 2004 Oct 25;167(2):327-37. doi: 10.1083/jcb.200403091. J Cell Biol. 2004. PMID: 15504914 Free PMC article.
-
Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association.J Cell Biol. 1998 Feb 9;140(3):647-57. doi: 10.1083/jcb.140.3.647. J Cell Biol. 1998. PMID: 9456324 Free PMC article.
-
Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins.J Cell Biol. 1999 Jun 28;145(7):1497-509. doi: 10.1083/jcb.145.7.1497. J Cell Biol. 1999. PMID: 10385528 Free PMC article.
-
Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins.J Biol Chem. 1999 Dec 3;274(49):34507-10. doi: 10.1074/jbc.274.49.34507. J Biol Chem. 1999. PMID: 10574907 Review. No abstract available.
-
ERM proteins: from cellular architecture to cell signaling.Biol Cell. 2000 Aug;92(5):305-16. doi: 10.1016/s0248-4900(00)01078-9. Biol Cell. 2000. PMID: 11071040 Review.
Cited by
-
Astrocytic LRRK2 Controls Synaptic Connectivity via Regulation of ERM Phosphorylation.bioRxiv [Preprint]. 2024 Aug 28:2023.04.09.536178. doi: 10.1101/2023.04.09.536178. bioRxiv. 2024. PMID: 39253496 Free PMC article. Preprint.
-
Gem associates with Ezrin and acts via the Rho-GAP protein Gmip to down-regulate the Rho pathway.Mol Biol Cell. 2007 Apr;18(4):1242-52. doi: 10.1091/mbc.e06-06-0510. Epub 2007 Jan 31. Mol Biol Cell. 2007. PMID: 17267693 Free PMC article.
-
Two Sides of the Coin: Ezrin/Radixin/Moesin and Merlin Control Membrane Structure and Contact Inhibition.Int J Mol Sci. 2019 Apr 23;20(8):1996. doi: 10.3390/ijms20081996. Int J Mol Sci. 2019. PMID: 31018575 Free PMC article. Review.
-
Plasticity in astrocyte subpopulations regulates heroin relapse.Sci Adv. 2022 Aug 12;8(32):eabo7044. doi: 10.1126/sciadv.abo7044. Epub 2022 Aug 10. Sci Adv. 2022. PMID: 35947652 Free PMC article.
-
Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway.J Cell Biol. 2008 Apr 21;181(2):335-50. doi: 10.1083/jcb.200705060. J Cell Biol. 2008. PMID: 18426979 Free PMC article.
References
-
- Andréoli C., Martin M., Le Borgne R., Reggio H., Mangeat P. Ezrin has properties to self-associate at the plasma membrane. J. Cell Sci. 1994;107:2509–2521. - PubMed
-
- Berryman M., Franck Z., Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 1993;105:1025–1043. - PubMed
-
- Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr. Opin. Cell Biol. 1999;11:109–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

