V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies
- PMID: 10888615
- PMCID: PMC112193
- DOI: 10.1128/jvi.74.15.6769-6776.2000
V2 loop glycosylation of the human immunodeficiency virus type 1 SF162 envelope facilitates interaction of this protein with CD4 and CCR5 receptors and protects the virus from neutralization by anti-V3 loop and anti-CD4 binding site antibodies
Abstract
We examined the role of asparagine-linked glycosylation of the V2 loop of the human immunodeficiency virus (HIV) SF162 envelope on viral replication potential and neutralization susceptibility. We report that the asparagines located at the amino- and carboxy-terminal sites (at positions 154 and 195, respectively), as well as within the V2 loop of the SF162 envelope (at position 186), are glycosylated during in vitro replication of this virus in human peripheral blood mononuclear cells. Our studies indicate that glycosylation of the V2 loop, in particular at its base, facilitates the interaction of the HIV envelope with the CD4 and CCR5 receptor molecules present on the surface of target cells and affects viral replication kinetics in a cell type-dependent manner. In cells expressing high numbers of receptor molecules on their surfaces, the SF162-derived V2 loop-deglycosylated mutant viruses replicate as efficiently as the parental SF162 virus, while in cells expressing small numbers of receptor molecules, the mutant viruses replicate with markedly reduced efficiency. In addition to expanding the viral tropism, V2 loop glycosylation at the three sites examined prevents neutralization by anti-CD4 binding site antibodies. In contrast, glycosylation at the amino- and carboxy-terminal sites of the V2 loop but not within the loop itself offers protection against anti-V3 loop antibodies. Thus, the epitopes masked by the sugar molecules present on the three glycosylation sites examined are not identical but overlap.
Figures
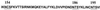

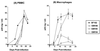
Similar articles
-
N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies.J Virol. 2004 Apr;78(7):3279-95. doi: 10.1128/jvi.78.7.3279-3295.2004. J Virol. 2004. PMID: 15016849 Free PMC article.
-
Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity.J Virol. 2006 Oct;80(19):9586-98. doi: 10.1128/JVI.00141-06. J Virol. 2006. PMID: 16973562 Free PMC article.
-
V3 loop truncations in HIV-1 envelope impart resistance to coreceptor inhibitors and enhanced sensitivity to neutralizing antibodies.PLoS Pathog. 2007 Aug 24;3(8):e117. doi: 10.1371/journal.ppat.0030117. PLoS Pathog. 2007. PMID: 17722977 Free PMC article.
-
Synthetic peptides for study of human immunodeficiency virus infection.Appl Biochem Biotechnol. 2002 Jul-Dec;102-103(1-6):41-7. doi: 10.1385/abab:102-103:1-6:041. Appl Biochem Biotechnol. 2002. PMID: 12396109 Review.
-
CD4-independent infection of HIV and SIV: implications for envelope conformation and cell tropism in vivo.AIDS. 2003;17 Suppl 4:S35-43. doi: 10.1097/00002030-200317004-00004. AIDS. 2003. PMID: 15080178 Review. No abstract available.
Cited by
-
Variable loop glycan dependency of the broad and potent HIV-1-neutralizing antibodies PG9 and PG16.J Virol. 2010 Oct;84(20):10510-21. doi: 10.1128/JVI.00552-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686044 Free PMC article.
-
Turnover of env variable region 1 and 2 genotypes in subjects with late-stage human immunodeficiency virus type 1 infection.J Virol. 2003 Jun;77(12):6811-22. doi: 10.1128/jvi.77.12.6811-6822.2003. J Virol. 2003. PMID: 12768001 Free PMC article.
-
Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate.J Virol. 2003 Oct;77(20):11244-59. doi: 10.1128/jvi.77.20.11244-11259.2003. J Virol. 2003. PMID: 14512572 Free PMC article.
-
N-linked glycosylation sites adjacent to and within the V1/V2 and the V3 loops of dualtropic human immunodeficiency virus type 1 isolate DH12 gp120 affect coreceptor usage and cellular tropism.J Virol. 2001 Jul;75(13):5998-6006. doi: 10.1128/JVI.75.13.5998-6006.2001. J Virol. 2001. PMID: 11390601 Free PMC article.
-
Addition of a single gp120 glycan confers increased binding to dendritic cell-specific ICAM-3-grabbing nonintegrin and neutralization escape to human immunodeficiency virus type 1.J Virol. 2002 Oct;76(20):10299-306. doi: 10.1128/jvi.76.20.10299-10306.2002. J Virol. 2002. PMID: 12239306 Free PMC article.
References
-
- Back N K T, Smit L, De Jong J-J, Keulen W, Schutten M, Goudsmit J, Tersmette M. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology. 1994;199:431–438. - PubMed
-
- Botarelli P, Houlden B A, Haigwood N L, Servis C, Montagna D, Abrignani S. N-glycosylation of HIV-gp120 may constrain recognition by T lymphocytes. J Immunol. 1991;147:3128–3132. - PubMed
-
- Cheng-Mayer C, Brown A, Harouse J, Luciw P A, Mayer A J. Selection for neutralization resistance of the simian/human immunodeficiency virus SHIVSF33A variant in vivo by virtue of sequence changes in the extracellular envelope glycoprotein that modify N-linked glycosylation. J Virol. 1999;73:5294–5300. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

