Need for an external proficiency testing program for cytokines, chemokines, and plasma markers of immune activation
- PMID: 10882648
- PMCID: PMC95910
- DOI: 10.1128/CDLI.7.4.540-548.2000
Need for an external proficiency testing program for cytokines, chemokines, and plasma markers of immune activation
Abstract
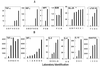
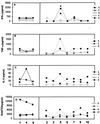
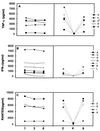
An external evaluation program for measuring the performance of laboratories testing for cytokines and immune activation markers in biological fluids was developed. Cytokines, chemokines, soluble cytokine receptors, and other soluble markers of immune activation (CSM) were measured in plasma from a healthy human immunodeficiency virus (HIV)-seronegative reference population and from HIV-seropositive individuals as well as in supernatant fluids from in vitro-stimulated human immune cells. The 14 components measured were tumor necrosis factor (TNF) alpha, gamma interferon, interleukin-1 (IL-1), IL-2, IL-4, IL-6, IL-10, Rantes, MIP-Ia, MIP-Ibeta, soluble TNF receptor II, soluble IL-2 receptor alpha, beta(2)-microglobulin, and neopterin. Twelve laboratories associated with the Adult and Pediatric AIDS Clinical Trial Groups participated in the study. The performance features that were evaluated included intralaboratory variability, interlaboratory variability, comparison of reagent sources, and ability to detect CSM in the plasma of normal subjects as well as the changes occurring in disease. The principal findings were as follows: (i) on initial testing, i.e., before participating in the program, laboratories frequently differed markedly in their analytic results; (ii) the quality of testing of a CSM in individual participating laboratories could be assessed; (iii) most commercial kits allowed distinction between normal and abnormal plasma CSM levels and between supernatants of stimulated and unstimulated cells; (iv) different sources of reagents and reference standards frequently provided different absolute values; (v) inexperienced laboratories can benefit from participating in the program; (vi) laboratory performance improved during active participation in the program; and (vii) comparability between analyses conducted at different sites can be ensured by an external proficiency testing program.
Figures

Similar articles
-
Levels of cytokines and immune activation markers in plasma in human immunodeficiency virus infection: quality control procedures.Clin Diagn Lab Immunol. 1998 Nov;5(6):755-61. doi: 10.1128/CDLI.5.6.755-761.1998. Clin Diagn Lab Immunol. 1998. PMID: 9801330 Free PMC article.
-
Variables that affect assays for plasma cytokines and soluble activation markers.Clin Diagn Lab Immunol. 1999 Jan;6(1):89-95. doi: 10.1128/CDLI.6.1.89-95.1999. Clin Diagn Lab Immunol. 1999. PMID: 9874670 Free PMC article.
-
Stability of plasma levels of cytokines and soluble activation markers in patients with human immunodeficiency virus infection.J Infect Dis. 1999 Apr;179(4):843-8. doi: 10.1086/314673. J Infect Dis. 1999. PMID: 10068579
-
Development and implementation of a proficiency testing program for Luminex bead-based cytokine assays.J Immunol Methods. 2014 Jul;409:62-71. doi: 10.1016/j.jim.2014.04.011. Epub 2014 May 4. J Immunol Methods. 2014. PMID: 24801479 Free PMC article. Review.
-
Cytokines and HIV-1: interactions and clinical implications.Antivir Chem Chemother. 2001 May;12(3):133-50. doi: 10.1177/095632020101200301. Antivir Chem Chemother. 2001. PMID: 12959322 Review.
Cited by
-
Serum neopterin levels in patients with replicative and nonreplicative HBV carriers.BMC Infect Dis. 2006 Oct 31;6:157. doi: 10.1186/1471-2334-6-157. BMC Infect Dis. 2006. PMID: 17076882 Free PMC article.
-
Multisite comparison of high-sensitivity multiplex cytokine assays.Clin Vaccine Immunol. 2011 Aug;18(8):1229-42. doi: 10.1128/CVI.05032-11. Epub 2011 Jun 22. Clin Vaccine Immunol. 2011. PMID: 21697338 Free PMC article.
-
Can Biomarkers Advance HIV Research and Care in the Antiretroviral Therapy Era?J Infect Dis. 2018 Jan 30;217(4):521-528. doi: 10.1093/infdis/jix586. J Infect Dis. 2018. PMID: 29165684 Free PMC article. Review.
-
Development of a proficiency testing program for the HIV-1 BED incidence assay in China.Sci Rep. 2014 Mar 28;4:4512. doi: 10.1038/srep04512. Sci Rep. 2014. PMID: 24676229 Free PMC article.
-
Measurement of Circulating Cytokines and Immune-Activation Markers by Multiplex Technology in the Clinical Setting: What Are We Really Measuring?For Immunopathol Dis Therap. 2015;6(1-2):19-22. doi: 10.1615/ForumImmunDisTher.2015014162. For Immunopathol Dis Therap. 2015. PMID: 28286693 Free PMC article.
References
-
- Bienvenu J, Coulon L, Doche C, Gutowski M C, Grau G. Analytical performances of commercial ELISA kits for IL-2, IL-6 and TNFα. A WHO study. Eur Cytokine Netw. 1993;4:447–451. - PubMed
-
- DeKossodo S, Houba V, Grau G E WHO Collaborative Study Group. Assaying tumor necrosis factor concentration in human serum. A WHO international collaborative study. J Immunol Methods. 1995;182:107–114. - PubMed
-
- Fahey J L, Taylor J M G, Manna B, Nishanian P, Aziz N, Giorgi J V, Detels R. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

