Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3
- PMID: 10880530
- PMCID: PMC1887707
- DOI: 10.1084/jem.192.1.99
Negative regulation of T cell proliferation and interleukin 2 production by the serine threonine kinase GSK-3
Abstract
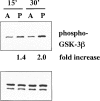
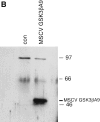
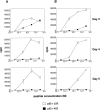
Glycogen synthase kinase (GSK)-3 is a protein serine/threonine kinase that regulates differentiation and cell fate in a variety of organisms. This study examined the role of GSK-3 in antigen-specific T cell responses. Using resting T cells from P14 T cell receptor (TCR)-transgenic mice (specific for the lymphocytic choriomeningitis virus and H-2D(b)), we demonstrated that GSK-3beta was inactivated by serine phosphorylation after viral peptide-specific stimulation in vitro. To further investigate the role of GSK-3, we have generated a retroviral vector that expresses a constitutively active form of GSK-3beta that has an alanine substitution at the regulatory amino acid, serine 9 (GSK-3betaA9). Retroviral transduction of P14 TCR-transgenic bone marrow stem cells, followed by reconstitution, led to the expression of GSK-3betaA9 in bone marrow chimeric mice. T cells from chimeric mice demonstrate a reduction in proliferation and interleukin (IL)-2 production. In contrast, in vitro assays done in the presence of the GSK-3 inhibitor lithium led to dramatically prolonged T cell proliferation and increased IL-2 production. Furthermore, in the presence of lithium, we show that nuclear factor of activated T cells (NF-AT)c remains in the nucleus after antigen-specific stimulation of T cells. Together, these data demonstrate that GSK-3 negatively regulates the duration of T cell responses.
Figures


Similar articles
-
The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase.J Immunol. 1999 Aug 15;163(4):1894-905. J Immunol. 1999. PMID: 10438924
-
Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation.Nature. 2000 Jul 6;406(6791):86-90. doi: 10.1038/35017574. Nature. 2000. PMID: 10894547
-
Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation.J Biol Chem. 1998 Jun 5;273(23):14315-21. doi: 10.1074/jbc.273.23.14315. J Biol Chem. 1998. PMID: 9603939
-
Dysregulation of glycogen synthase kinase-3beta signaling in hepatocellular carcinoma cells.Hepatology. 2002 Dec;36(6):1528-36. doi: 10.1053/jhep.2002.37192. Hepatology. 2002. PMID: 12447879
-
Pathobiology and Therapeutic Relevance of GSK-3 in Chronic Hematological Malignancies.Cells. 2022 May 31;11(11):1812. doi: 10.3390/cells11111812. Cells. 2022. PMID: 35681507 Free PMC article. Review.
Cited by
-
Loss of GTPase of immunity-associated protein 5 (Gimap5) promotes pathogenic CD4+ T-cell development and allergic airway disease.J Allergy Clin Immunol. 2019 Jan;143(1):245-257.e6. doi: 10.1016/j.jaci.2018.10.018. Epub 2018 Oct 25. J Allergy Clin Immunol. 2019. PMID: 30616774 Free PMC article.
-
Suppressive regulatory T cell activity is potentiated by glycogen synthase kinase 3{beta} inhibition.J Biol Chem. 2010 Oct 22;285(43):32852-32859. doi: 10.1074/jbc.M110.150904. Epub 2010 Aug 20. J Biol Chem. 2010. PMID: 20729209 Free PMC article.
-
Altered Germinal-Center Metabolism in B Cells in Autoimmunity.Metabolites. 2022 Jan 5;12(1):40. doi: 10.3390/metabo12010040. Metabolites. 2022. PMID: 35050162 Free PMC article. Review.
-
Gimap5-dependent inactivation of GSK3β is required for CD4+ T cell homeostasis and prevention of immune pathology.Nat Commun. 2018 Jan 30;9(1):430. doi: 10.1038/s41467-018-02897-7. Nat Commun. 2018. PMID: 29382851 Free PMC article.
-
Lithium promotes neural precursor cell proliferation: evidence for the involvement of the non-canonical GSK-3β-NF-AT signaling.Cell Biosci. 2011 May 3;1(1):18. doi: 10.1186/2045-3701-1-18. Cell Biosci. 2011. PMID: 21711903 Free PMC article.
References
-
- Qian D., Weiss A. T cell antigen receptor signal transduction. Curr. Opin. Cell Biol. 1997;9:205–212. - PubMed
-
- Alberola-Ila J., Takaki S., Kerner J.D., Perlmutter R.M. Differential signalling by lymphocyte antigen receptors. Annu. Rev. Immunol. 1997;15:125–154. - PubMed
-
- Rao A., Luo C., Hogan P.G. Transcription factors of the NFAT familyregulation and function. Annu. Rev. Immunol. 1997;15:707–747. - PubMed
-
- Woodgett J.R. Regulation and functions of the glycogen synthase kinase-3 subfamily. Cancer Biol. 1994;5:269–275. - PubMed
-
- Welsh G.I., Wilson C., Proud C.G. GSK3a SHAGGY frog story. Cell Biol. 1996;6:274–279. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

