Cowpea mosaic virus infection induces a massive proliferation of endoplasmic reticulum but not Golgi membranes and is dependent on de novo membrane synthesis
- PMID: 10864669
- PMCID: PMC112165
- DOI: 10.1128/jvi.74.14.6556-6563.2000
Cowpea mosaic virus infection induces a massive proliferation of endoplasmic reticulum but not Golgi membranes and is dependent on de novo membrane synthesis
Abstract
Replication of cowpea mosaic virus (CPMV) is associated with small membranous vesicles that are induced upon infection. The effect of CPMV replication on the morphology and distribution of the endomembrane system in living plant cells was studied by expressing green fluorescent protein (GFP) targeted to the endoplasmic reticulum (ER) and the Golgi membranes. CPMV infection was found to induce an extensive proliferation of the ER, whereas the distribution and morphology of the Golgi stacks remained unaffected. Immunolocalization experiments using fluorescence confocal microscopy showed that the proliferated ER membranes were closely associated with the electron-dense structures that contain the replicative proteins encoded by RNA1. Replication of CPMV was strongly inhibited by cerulenin, an inhibitor of de novo lipid synthesis, at concentrations where the replication of the two unrelated viruses alfalfa mosaic virus and tobacco mosaic virus was largely unaffected. These results suggest that proliferating ER membranes produce the membranous vesicles formed during CPMV infection and that this process requires continuous lipid biosynthesis.
Figures

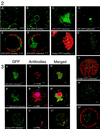

Similar articles
-
Coalescence of the sites of cowpea mosaic virus RNA replication into a cytopathic structure.J Virol. 2002 Jun;76(12):6235-43. doi: 10.1128/jvi.76.12.6235-6243.2002. J Virol. 2002. PMID: 12021357 Free PMC article.
-
Cowpea mosaic virus 32- and 60-kilodalton replication proteins target and change the morphology of endoplasmic reticulum membranes.J Virol. 2002 Jun;76(12):6293-301. doi: 10.1128/jvi.76.12.6293-6301.2002. J Virol. 2002. PMID: 12021362 Free PMC article.
-
Intracellular localization of the peanut clump virus replication complex in tobacco BY-2 protoplasts containing green fluorescent protein-labeled endoplasmic reticulum or Golgi apparatus.J Virol. 2002 Jan;76(2):865-74. doi: 10.1128/jvi.76.2.865-874.2002. J Virol. 2002. PMID: 11752175 Free PMC article.
-
Microscopic morphology and the origins of the membrane maturation model of Golgi apparatus function.Int Rev Cytol. 2007;262:191-218. doi: 10.1016/S0074-7696(07)62004-X. Int Rev Cytol. 2007. PMID: 17631189 Review.
-
Biogenesis of the plant Golgi apparatus.Biochem Soc Trans. 2010 Jun;38(3):761-7. doi: 10.1042/BST0380761. Biochem Soc Trans. 2010. PMID: 20491662 Review.
Cited by
-
Cell biological and functional characterization of the vaccinia virus F10 kinase: implications for the mechanism of virion morphogenesis.J Virol. 2005 Feb;79(4):2171-90. doi: 10.1128/JVI.79.4.2171-2190.2005. J Virol. 2005. PMID: 15681420 Free PMC article.
-
Chloroplast phosphoglycerate kinase is involved in the targeting of Bamboo mosaic virus to chloroplasts in Nicotiana benthamiana plants.Plant Physiol. 2013 Dec;163(4):1598-608. doi: 10.1104/pp.113.229666. Epub 2013 Oct 23. Plant Physiol. 2013. PMID: 24154620 Free PMC article.
-
Evidence that insertion of Tomato ringspot nepovirus NTB-VPg protein in endoplasmic reticulum membranes is directed by two domains: a C-terminal transmembrane helix and an N-terminal amphipathic helix.J Virol. 2005 Sep;79(18):11752-65. doi: 10.1128/JVI.79.18.11752-11765.2005. J Virol. 2005. PMID: 16140753 Free PMC article.
-
Coalescence of the sites of cowpea mosaic virus RNA replication into a cytopathic structure.J Virol. 2002 Jun;76(12):6235-43. doi: 10.1128/jvi.76.12.6235-6243.2002. J Virol. 2002. PMID: 12021357 Free PMC article.
-
Subcellular localization of host and viral proteins associated with tobamovirus RNA replication.EMBO J. 2003 Jan 15;22(2):344-53. doi: 10.1093/emboj/cdg033. EMBO J. 2003. PMID: 12514140 Free PMC article.
References
-
- Bienz K, Egger D, Pasamontes L. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology. 1987;160:220–226. - PubMed
-
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J. 1998;15:441–447. - PubMed
-
- Boevink P, Santa Cruz S, Hawes C, Harris N, Oparka K J. Virus-mediated delivery of the green fluorescent protein to the endoplasmic reticulum of plant cells. Plant J. 1996;10:935–941.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

